We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Jennifer Taylor/Sandbox 3
From Proteopedia
< User:Jennifer Taylor(Difference between revisions)
m |
m |
||
| (7 intermediate revisions not shown.) | |||
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
| - | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as <scene name='78/787193/3h04_n_to_c_rainbow/1'>3H04</scene>, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. ''In silico'' enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural and protein sequence analysis suggests PDB entries 1AZW and 1YSC | + | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as <scene name='78/787193/3h04_n_to_c_rainbow/1'>3H04</scene>, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. ''In silico'' enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural and protein sequence analysis suggests PDB entries <scene name='78/787193/1azw/1'>1AZW</scene> and <scene name='78/787193/1ysc/1'>1YSC</scene> , respectively, are most similar to 3H04; however 1TAH and 3H04 share the most catalytic triad similarity. To investigate the lipase activity of 3H04 ''in vitro'', recombinant his-tagged protein was isolated from E. coli. |
Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | ||
| Line 14: | Line 14: | ||
==Hypothesis== | ==Hypothesis== | ||
| - | In the beginning, we predicted that our protein was an esterase, because originally our most promising homologs were an aminopeptidase and an esterase. And even though the most hits from our Promol search were enzyme class 3.4 (aminopeptidases), no further information was found about the catalytic triads of these hits. We decided to use a homolog in E.C. 3.1 (esterase) because there was information about their catalytic triads. We knew that our protein is a hydrolase, and we believe that 3H04 is characterized as an esterase (3.1), instead of an aminopeptidase (3.4). Even though the 3.4 category proteins had the most motif structure similarity in Promol, we chose 1TAH (a lipase) as our target homolog. 1TAH is a characterized protein and has a | + | In the beginning, we predicted that our protein was an esterase, because originally our most promising homologs were an aminopeptidase and an esterase. And even though the most hits from our Promol search were enzyme class 3.4 (aminopeptidases), no further information was found about the catalytic triads of these hits. We decided to use a homolog in E.C. 3.1 (esterase) because there was information about their catalytic triads. We knew that our protein is a hydrolase, and we believe that 3H04 is characterized as an esterase (3.1), instead of an aminopeptidase (3.4). Even though the 3.4 category proteins had the most motif structure similarity in Promol, we chose <scene name='78/787193/1tah/1'>1TAH</scene> (a lipase) as our target homolog. 1TAH is a characterized protein and has a similar catalytic triad to what we predict for our protein (3H04). When we aligned the two catalytic triads <scene name='78/787193/Catalytic_triad_of_1tah/2'>1TAH</scene> and <scene name='78/787193/Catalytic_triad_3h04/1'>3H04</scene>, they had an RMS of 0.016. In the beginning we were looking for a homolog with more protein sequence and structure similarity, but because neither protein sequence or structure determine the function of 3H04, we decided that those two factors matter much less than the catalytic triad of the protein. So, we used Promol to find what the catalytic triad of 3H04 is and we used 1TAH as our target homolog for the catalytic triad. We predict, because the RMS was so low, that 3H04 and 1TAH will be having very similar enzymatic functions. |
== Methods == | == Methods == | ||
| Line 30: | Line 30: | ||
== Conclusion == | == Conclusion == | ||
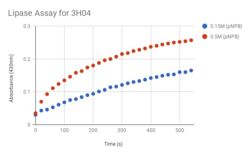
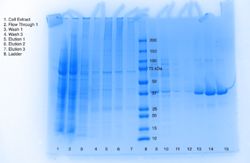
| - | We've concluded that our protein is a lipase, and because we expressed our protein through E.coli, we believe that 3H04 functions in the lower intestine of warm blooded organisms. | + | We've concluded that our protein is a lipase, and because we expressed our protein through E.coli, we believe that 3H04 functions in the lower intestine of warm blooded organisms. The blue dots in Figure 1 represent the pNPB at a lower concentration than the red dots. The blue dots represent the reaction happening at 0.15M and the red dots represent the reaction with a concentration of 0.5M, both concentrations showed positive results of a reaction, however the expected curve of the graph was not as steep as predicted with the original concentration of 0.15M. The concentration was increased to increase the curve of the graph so that the expected curve was attained. The stained coomaise gel in Figure 2 shows that our protein was being expressed through the bacterial cells, around the correct expected molecular weight. This protein, expressed, purified, and now characterized is in the enzyme class lipase. |
| - | + | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | < | + | Zhang, R., Tesar, C., Sather, A., Clancy, S., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)The crystal structure of the protein with unknown function from Staphylococcus aureus subsp. aureus Mu50 |
| + | The Pfam protein families database: towards a more sustainable future <https://nar.oxfordjournals.org/content/44/D1/D279.long>: R.D. Finn, P. Coggill, R.Y. Eberhardt, S.R. Eddy, J. Mistry, A.L. Mitchell, S.C. Potter, M. Punta, M. Qureshi, A. Sangrador-Vegas, G.A. Salazar, J. Tate, A. Bateman | ||
| + | Nucleic Acids Research (2016) Database Issue 44:D279-D285 | ||
| + | PyMOL | ||
| + | The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC. | ||
Current revision
3H04 Test Page
| |||||||||||
References
Zhang, R., Tesar, C., Sather, A., Clancy, S., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)The crystal structure of the protein with unknown function from Staphylococcus aureus subsp. aureus Mu50 The Pfam protein families database: towards a more sustainable future <https://nar.oxfordjournals.org/content/44/D1/D279.long>: R.D. Finn, P. Coggill, R.Y. Eberhardt, S.R. Eddy, J. Mistry, A.L. Mitchell, S.C. Potter, M. Punta, M. Qureshi, A. Sangrador-Vegas, G.A. Salazar, J. Tate, A. Bateman Nucleic Acids Research (2016) Database Issue 44:D279-D285 PyMOL The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.