User:Jennifer Taylor/Sandbox 3
From Proteopedia
< User:Jennifer Taylor(Difference between revisions)
m |
|||
| (One intermediate revision not shown.) | |||
| Line 6: | Line 6: | ||
==Introduction== | ==Introduction== | ||
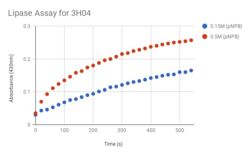
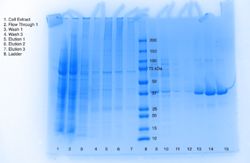
| - | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as <scene name='78/787193/3h04_n_to_c_rainbow/1'>3H04</scene>, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. ''In silico'' enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural and protein sequence analysis suggests PDB entries <scene name='78/787193/1azw/1'>1AZW</scene> and <scene name='78/787193/1ysc/1'>1YSC</scene> | + | Beginning in 2000, the National Institutes of Health’s Protein Structure Initiative was a fifteen-year program that worked to solve the structures of many proteins with known DNA sequences. The present study analyzes one such protein, known as <scene name='78/787193/3h04_n_to_c_rainbow/1'>3H04</scene>, a predicted alpha/beta hydrolase found in Escherichia Coli. The specific objective of this study is to functionally characterize 3H04 based on structural analysis of the protein. ''In silico'' enzyme characterization for 3H04 was carried out using BLAST, Pfam, and Dali servers, followed by active site analysis using the PyMOL plug-in ProMOL. This structural and protein sequence analysis suggests PDB entries <scene name='78/787193/1azw/1'>1AZW</scene> and <scene name='78/787193/1ysc/1'>1YSC</scene> , respectively, are most similar to 3H04; however 1TAH and 3H04 share the most catalytic triad similarity. To investigate the lipase activity of 3H04 ''in vitro'', recombinant his-tagged protein was isolated from E. coli. |
Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | Proteins are macromolecules that are present in nearly every earthly organism and represent a vast array of functions. Scientists have been able to structurally solve proteins at a faster rate than they have been able to identify the functions of these same proteins. In an effort to close this gap, the National Institutes of Health launched the Protein Structure Initiative, which was a fifteen-year program, starting in 2000, that worked to solve the structures of many proteins with known DNA sequences. However, there are still hundreds proteins housed within the Protein Data Bank that have unidentified functions. Researchers are continuing to advocate for further analysis of these proteins through the development of BASIL modules that explain how to use ProMOL to analyzes the structural similarities between the active sites of proteins. | ||
| Line 35: | Line 35: | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | < | + | Zhang, R., Tesar, C., Sather, A., Clancy, S., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)The crystal structure of the protein with unknown function from Staphylococcus aureus subsp. aureus Mu50 |
| + | The Pfam protein families database: towards a more sustainable future <https://nar.oxfordjournals.org/content/44/D1/D279.long>: R.D. Finn, P. Coggill, R.Y. Eberhardt, S.R. Eddy, J. Mistry, A.L. Mitchell, S.C. Potter, M. Punta, M. Qureshi, A. Sangrador-Vegas, G.A. Salazar, J. Tate, A. Bateman | ||
| + | Nucleic Acids Research (2016) Database Issue 44:D279-D285 | ||
| + | PyMOL | ||
| + | The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC. | ||
Current revision
3H04 Test Page
| |||||||||||
References
Zhang, R., Tesar, C., Sather, A., Clancy, S., Joachimiak, A., Midwest Center for Structural Genomics (MCSG)The crystal structure of the protein with unknown function from Staphylococcus aureus subsp. aureus Mu50 The Pfam protein families database: towards a more sustainable future <https://nar.oxfordjournals.org/content/44/D1/D279.long>: R.D. Finn, P. Coggill, R.Y. Eberhardt, S.R. Eddy, J. Mistry, A.L. Mitchell, S.C. Potter, M. Punta, M. Qureshi, A. Sangrador-Vegas, G.A. Salazar, J. Tate, A. Bateman Nucleic Acids Research (2016) Database Issue 44:D279-D285 PyMOL The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC.