AppA protein BLUF domain
From Proteopedia
(Difference between revisions)
m (Appa protein BLUF domain moved to AppA protein BLUF domain) |
|||
| (7 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
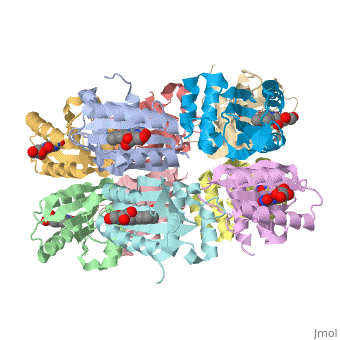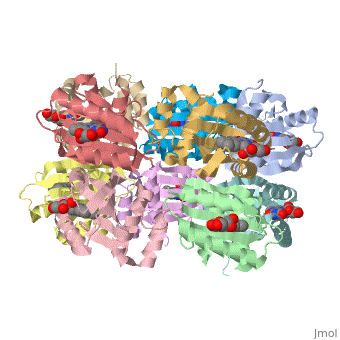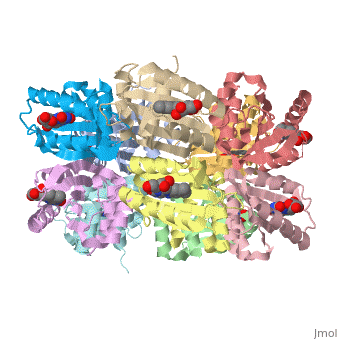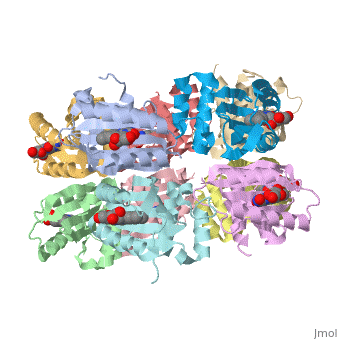
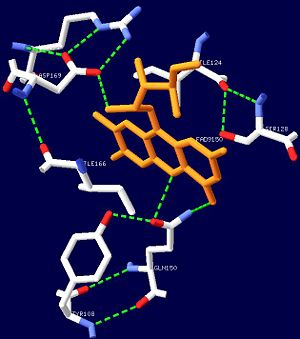
<StructureSection load='1x0p' size='350' side='right' caption='Structure of BLUF domain protein complex with FAD (PDB code [[1x0p]]).' scene=''> | <StructureSection load='1x0p' size='350' side='right' caption='Structure of BLUF domain protein complex with FAD (PDB code [[1x0p]]).' scene=''> | ||
=Introduction= | =Introduction= | ||
| - | + | '''AppA''' is an anti repressor in which blue light excitation induces a long-lived red-shift absorbance in photosystem synthesis<ref>PMID:12230978</ref> . AppA contains sensor for '''blue light using''' [http://en.wikipedia.org/wiki/Flavin_adenine_dinucleotide FAD] '''(BLUF) domains''' are one class of photoreceptor family that utilizes a flavin [http://en.wikipedia.org/wiki/Chromophore chromophore]<ref name ="one">PMID: 15876364</ref>. The other two classes include [http://en.wikipedia.org/wiki/Phototropin phototropins] (LOV) and [http://en.wikipedia.org/wiki/Cryptochrome cryptochromes]<ref name="two">PMID: 14730990</ref>. The BLUF domain was first discovered in [http://microbewiki.kenyon.edu/index.php/Rhodobacter ''Rhodobacter sphaeroides''] as the blue light photoreceptor involved in the repression of photosynthesis genes in '''AppA''' protein<ref name="one" /><ref name ="three">PMID: 12230978</ref>. The BLUF domain is known to exist in many bacteria, including cyanobacteria. | |
One unique photosensing property of BLUF domain is a light induced spectral shift in the flavin absorption spectrum where the wavelength is longer by approximately 10nm<ref name="one" />. In AppA this shift occurs quickly upon illumination and is slowly reversed upon return to darkness. This spectral shift is not well understood and is not observed in other flavin binding photoreceptors. There have been two proposed models to explain the spectral shift observed in BLUF containing proteins: π-π stacking between the isoalloxazine ring of flavin and the phenol sidechain of a conserved tyrosine residue or protonation and deprotonation of the flavin ring coupled directly or indirectly with the conserved tyrosine residue<ref name ="four">PMID: 14556317</ref><ref name ="five">PMID: 15659451</ref>. These two models are based upon the conserved tyrosine residue Tyr9 in the T110078 protein. | One unique photosensing property of BLUF domain is a light induced spectral shift in the flavin absorption spectrum where the wavelength is longer by approximately 10nm<ref name="one" />. In AppA this shift occurs quickly upon illumination and is slowly reversed upon return to darkness. This spectral shift is not well understood and is not observed in other flavin binding photoreceptors. There have been two proposed models to explain the spectral shift observed in BLUF containing proteins: π-π stacking between the isoalloxazine ring of flavin and the phenol sidechain of a conserved tyrosine residue or protonation and deprotonation of the flavin ring coupled directly or indirectly with the conserved tyrosine residue<ref name ="four">PMID: 14556317</ref><ref name ="five">PMID: 15659451</ref>. These two models are based upon the conserved tyrosine residue Tyr9 in the T110078 protein. | ||
| Line 26: | Line 26: | ||
Overall, the main function of the BLUF domain is to detect and respond to blue light. More specifically, in ''R. sphaeroides'', the BLUF domain is a blue light photo receptor involved in repressing the photosynthesis genes at the N-terminal region of the AppA protein<ref name="three" />. In ''E. gracilis'', the BLUF domain of PAC complexes serves as a blue light receptor in photophobic responses<ref name="six" />. | Overall, the main function of the BLUF domain is to detect and respond to blue light. More specifically, in ''R. sphaeroides'', the BLUF domain is a blue light photo receptor involved in repressing the photosynthesis genes at the N-terminal region of the AppA protein<ref name="three" />. In ''E. gracilis'', the BLUF domain of PAC complexes serves as a blue light receptor in photophobic responses<ref name="six" />. | ||
</StructureSection> | </StructureSection> | ||
| - | =3D structures of | + | =3D structures of AppA= |
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | [[2byc]] | + | [[2byc]] – RsBlue light receptor BLUF + FMN - ''Rhodobacter sphaeroides''<br /> |
| - | [[2iyg]], [[2iyi]] | + | [[2bun]] – RsAppA BLUF domain + FAD – NMR <br /> |
| - | [[1x0p]] - BLUF - ''Thermosynechococcus elongatus''<br /> | + | [[2iyg]], [[2iyi]] – RsAppA BLUF domain (mutant) + FMN <br /> |
| - | + | [[4heh]] – RsAppA SCHIC domain <br /> | |
| + | [[4hh3]] – RsAppA SCHIC domain (mutant) + PpsR<br /> | ||
| + | [[4hh1]] – RsAppA + FMN<br /> | ||
| + | [[4hh0]] – RsAppA (mutant) + FMN<br /> | ||
| + | [[1x0p]] - BLUF + FAD - ''Thermosynechococcus elongatus''<br /> | ||
| + | [[1xoc]] – AppA + peptide - ''Bacillus subtilis''<br /> | ||
| + | [[4tsr]] – AppA (mutant) + inositol hexasulfate - ''Eschericia coli''<br /> | ||
=References= | =References= | ||
<references /> | <references /> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D structures of AppA
Updated on 13-May-2021
2byc – RsBlue light receptor BLUF + FMN - Rhodobacter sphaeroides
2bun – RsAppA BLUF domain + FAD – NMR
2iyg, 2iyi – RsAppA BLUF domain (mutant) + FMN
4heh – RsAppA SCHIC domain
4hh3 – RsAppA SCHIC domain (mutant) + PpsR
4hh1 – RsAppA + FMN
4hh0 – RsAppA (mutant) + FMN
1x0p - BLUF + FAD - Thermosynechococcus elongatus
1xoc – AppA + peptide - Bacillus subtilis
4tsr – AppA (mutant) + inositol hexasulfate - Eschericia coli
References
- ↑ Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002 Sep 6;110(5):613-23. PMID:12230978
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 Kita A, Okajima K, Morimoto Y, Ikeuchi M, Miki K. Structure of a cyanobacterial BLUF protein, Tll0078, containing a novel FAD-binding blue light sensor domain. J Mol Biol. 2005 May 27;349(1):1-9. Epub 2005 Apr 9. PMID:15876364 doi:10.1016/j.jmb.2005.03.067
- ↑ van der Horst MA, Hellingwerf KJ. Photoreceptor proteins, "star actors of modern times": a review of the functional dynamics in the structure of representative members of six different photoreceptor families. Acc Chem Res. 2004 Jan;37(1):13-20. PMID:14730990 doi:10.1021/ar020219d
- ↑ 4.0 4.1 Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002 Sep 6;110(5):613-23. PMID:12230978
- ↑ Laan W, van der Horst MA, van Stokkum IH, Hellingwerf KJ. Initial characterization of the primary photochemistry of AppA, a blue-light-using flavin adenine dinucleotide-domain containing transcriptional antirepressor protein from Rhodobacter sphaeroides: a key role for reversible intramolecular proton transfer from the flavin adenine dinucleotide chromophore to a conserved tyrosine? Photochem Photobiol. 2003 Sep;78(3):290-7. PMID:14556317
- ↑ Hasegawa K, Masuda S, Ono TA. Spectroscopic analysis of the dark relaxation process of a photocycle in a sensor of blue light using FAD (BLUF) protein Slr1694 of the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2005 Jan;46(1):136-46. Epub 2005 Jan 19. PMID:15659451 doi:10.1093/pcp/pci003
- ↑ 7.0 7.1 Iseki M, Matsunaga S, Murakami A, Ohno K, Shiga K, Yoshida K, Sugai M, Takahashi T, Hori T, Watanabe M. A blue-light-activated adenylyl cyclase mediates photoavoidance in Euglena gracilis. Nature. 2002 Feb 28;415(6875):1047-51. PMID:11875575 doi:10.1038/4151047a
- ↑ Yuan H, Anderson S, Masuda S, Dragnea V, Moffat K, Bauer C. Crystal structures of the Synechocystis photoreceptor Slr1694 reveal distinct structural states related to signaling. Biochemistry. 2006 Oct 24;45(42):12687-94. PMID:17042486 doi:10.1021/bi061435n
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Amanda Cookhouse, Jaime Prilusky