|
|
| (One intermediate revision not shown.) |
| Line 1: |
Line 1: |
| | | | |
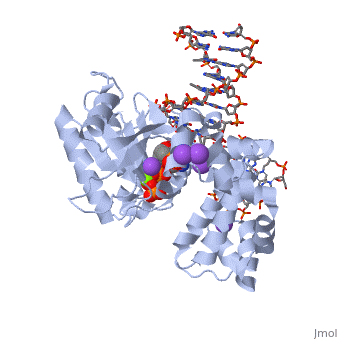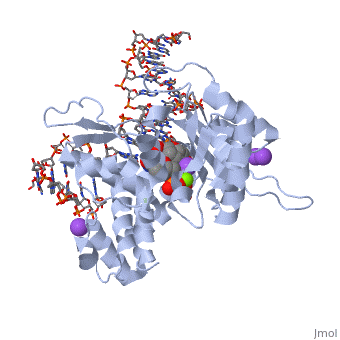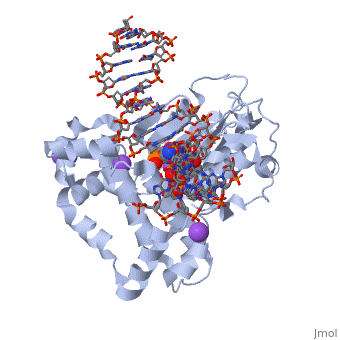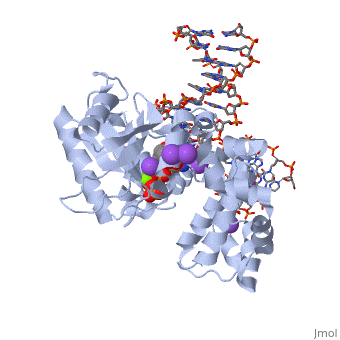
| | ==DNA Polymerase beta with a terminated gapped DNA substrate and ddCTP with sodium in the catalytic site== | | ==DNA Polymerase beta with a terminated gapped DNA substrate and ddCTP with sodium in the catalytic site== |
| - | <StructureSection load='2fmp' size='340' side='right' caption='[[2fmp]], [[Resolution|resolution]] 1.65Å' scene=''> | + | <StructureSection load='2fmp' size='340' side='right'caption='[[2fmp]], [[Resolution|resolution]] 1.65Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[2fmp]] is a 4 chain structure with sequence from [http://en.wikipedia.org/wiki/Human Human]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2FMP OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2FMP FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[2fmp]] is a 4 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2FMP OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=2FMP FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=DCT:2,3-DIDEOXYCYTIDINE+5-TRIPHOSPHATE'>DCT</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NA:SODIUM+ION'>NA</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 1.65Å</td></tr> |
| - | <tr id='NonStdRes'><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=DOC:2,3-DIDEOXYCYTIDINE-5-MONOPHOSPHATE'>DOC</scene></td></tr> | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=DCT:2,3-DIDEOXYCYTIDINE+5-TRIPHOSPHATE'>DCT</scene>, <scene name='pdbligand=DOC:2,3-DIDEOXYCYTIDINE-5-MONOPHOSPHATE'>DOC</scene>, <scene name='pdbligand=MG:MAGNESIUM+ION'>MG</scene>, <scene name='pdbligand=NA:SODIUM+ION'>NA</scene></td></tr> |
| - | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[2fmq|2fmq]], [[2fms|2fms]]</td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=2fmp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2fmp OCA], [https://pdbe.org/2fmp PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=2fmp RCSB], [https://www.ebi.ac.uk/pdbsum/2fmp PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=2fmp ProSAT]</span></td></tr> |
| - | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">POLB ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=9606 HUMAN])</td></tr>
| + | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2fmp FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2fmp OCA], [http://pdbe.org/2fmp PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=2fmp RCSB], [http://www.ebi.ac.uk/pdbsum/2fmp PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=2fmp ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/DPOLB_HUMAN DPOLB_HUMAN]] Repair polymerase that plays a key role in base-excision repair. Has 5'-deoxyribose-5-phosphate lyase (dRP lyase) activity that removes the 5' sugar phosphate and also acts as a DNA polymerase that adds one nucleotide to the 3' end of the arising single-nucleotide gap. Conducts 'gap-filling' DNA synthesis in a stepwise distributive fashion rather than in a processive fashion as for other DNA polymerases.<ref>PMID:9207062</ref> <ref>PMID:9572863</ref> <ref>PMID:11805079</ref> <ref>PMID:21362556</ref> | + | [https://www.uniprot.org/uniprot/DPOLB_HUMAN DPOLB_HUMAN] Repair polymerase that plays a key role in base-excision repair. Has 5'-deoxyribose-5-phosphate lyase (dRP lyase) activity that removes the 5' sugar phosphate and also acts as a DNA polymerase that adds one nucleotide to the 3' end of the arising single-nucleotide gap. Conducts 'gap-filling' DNA synthesis in a stepwise distributive fashion rather than in a processive fashion as for other DNA polymerases.<ref>PMID:9207062</ref> <ref>PMID:9572863</ref> <ref>PMID:11805079</ref> <ref>PMID:21362556</ref> |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 33: |
Line 31: |
| | | | |
| | ==See Also== | | ==See Also== |
| - | *[[DNA polymerase|DNA polymerase]] | + | *[[DNA polymerase 3D structures|DNA polymerase 3D structures]] |
| | *[[DNA polymerase beta|DNA polymerase beta]] | | *[[DNA polymerase beta|DNA polymerase beta]] |
| | == References == | | == References == |
| Line 39: |
Line 37: |
| | __TOC__ | | __TOC__ |
| | </StructureSection> | | </StructureSection> |
| - | [[Category: Human]] | + | [[Category: Homo sapiens]] |
| - | [[Category: Batra, V K]] | + | [[Category: Large Structures]] |
| - | [[Category: Beard, W A]] | + | [[Category: Batra VK]] |
| - | [[Category: Krahn, J M]] | + | [[Category: Beard WA]] |
| - | [[Category: Pedersen, L C]] | + | [[Category: Krahn JM]] |
| - | [[Category: Shock, D D]] | + | [[Category: Pedersen LC]] |
| - | [[Category: Wilson, S H]] | + | [[Category: Shock DD]] |
| - | [[Category: Nucleotidyl transferase]] | + | [[Category: Wilson SH]] |
| - | [[Category: Transferase-dna complex]]
| + | |
| Structural highlights
Function
DPOLB_HUMAN Repair polymerase that plays a key role in base-excision repair. Has 5'-deoxyribose-5-phosphate lyase (dRP lyase) activity that removes the 5' sugar phosphate and also acts as a DNA polymerase that adds one nucleotide to the 3' end of the arising single-nucleotide gap. Conducts 'gap-filling' DNA synthesis in a stepwise distributive fashion rather than in a processive fashion as for other DNA polymerases.[1] [2] [3] [4]
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
The molecular details of the nucleotidyl transferase reaction have remained speculative, as strategies to trap catalytic intermediates for structure determination utilize substrates lacking the primer terminus 3'-OH and catalytic Mg2+, resulting in an incomplete and distorted active site geometry. Since the geometric arrangement of these essential atoms will impact chemistry, structural insight into fidelity strategies has been hampered. Here, we present a crystal structure of a precatalytic complex of a DNA polymerase with bound substrates that include the primer 3'-OH and catalytic Mg2+. This catalytic intermediate was trapped with a nonhydrolyzable deoxynucleotide analog. Comparison with two new structures of DNA polymerase beta lacking the 3'-OH or catalytic Mg2+ is described. These structures provide direct evidence that both atoms are required to achieve a proper geometry necessary for an in-line nucleophilic attack of O3' on the alphaP of the incoming nucleotide.
Magnesium-induced assembly of a complete DNA polymerase catalytic complex.,Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH Structure. 2006 Apr;14(4):757-66. PMID:16615916[5]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Bennett RA, Wilson DM 3rd, Wong D, Demple B. Interaction of human apurinic endonuclease and DNA polymerase beta in the base excision repair pathway. Proc Natl Acad Sci U S A. 1997 Jul 8;94(14):7166-9. PMID:9207062
- ↑ Matsumoto Y, Kim K, Katz DS, Feng JA. Catalytic center of DNA polymerase beta for excision of deoxyribose phosphate groups. Biochemistry. 1998 May 5;37(18):6456-64. PMID:9572863 doi:10.1021/bi9727545
- ↑ DeMott MS, Beyret E, Wong D, Bales BC, Hwang JT, Greenberg MM, Demple B. Covalent trapping of human DNA polymerase beta by the oxidative DNA lesion 2-deoxyribonolactone. J Biol Chem. 2002 Mar 8;277(10):7637-40. Epub 2002 Jan 22. PMID:11805079 doi:10.1074/jbc.C100577200
- ↑ Parsons JL, Dianova II, Khoronenkova SV, Edelmann MJ, Kessler BM, Dianov GL. USP47 is a deubiquitylating enzyme that regulates base excision repair by controlling steady-state levels of DNA polymerase beta. Mol Cell. 2011 Mar 4;41(5):609-15. doi: 10.1016/j.molcel.2011.02.016. PMID:21362556 doi:10.1016/j.molcel.2011.02.016
- ↑ Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Magnesium-induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006 Apr;14(4):757-66. PMID:16615916 doi:http://dx.doi.org/10.1016/j.str.2006.01.011
|