We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Kdo-8-phosphate synthase
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
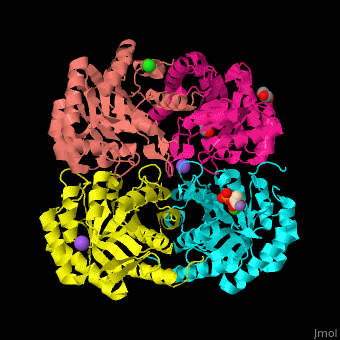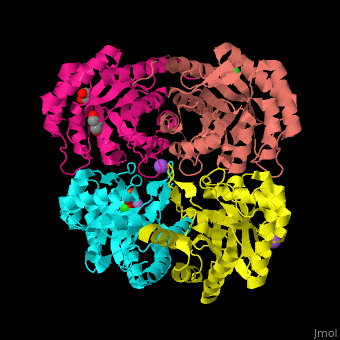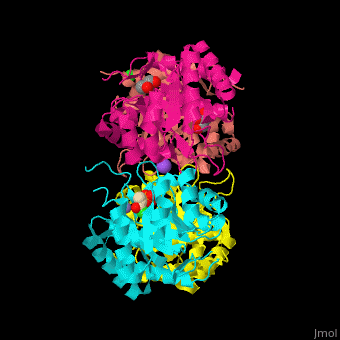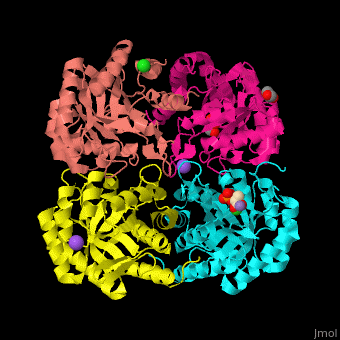
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' scene='52/525184/Cv/2' caption='Metallo Kdo-8-phosphate synthase tetramer complex with phosphoenolpyruvate, glycerol, Cl- (green), Na+ (large purple) and Mn+2 (small purple) ion, [[3fyp]]'> |
== Function == | == Function == | ||
| - | '''Kdo-8-phosphate synthase''' (KPS) catalyzes the formation of the phosphorylated rare sugar Kdo-8-phosphate (Kdo-8-P) from phosphoenolpyruvate (PEP) and D-arabinose-5-phosphate (A5P). Some bacteria like ''Aquifex aeolicus'' have '''Metallo KPS''' which is a metal-requiring enzyme while others like ''E.coli'' have a '''Non-metallo KPS''' which does not require a divalent cation for its activity. Kdo-8-P is found in cell walls of higher plants and some green algae<ref>PMID:11904225</ref>. | + | '''Kdo-8-phosphate synthase''' or '''2-dehydro-3-deoxyphosphooctonate aldolase''' (KPS) catalyzes the formation of the phosphorylated rare sugar Kdo-8-phosphate (Kdo-8-P) from phosphoenolpyruvate (PEP) and D-arabinose-5-phosphate (A5P). Some bacteria like ''Aquifex aeolicus'' have '''Metallo KPS''' which is a metal-requiring enzyme while others like ''E.coli'' have a '''Non-metallo KPS''' which does not require a divalent cation for its activity. Kdo-8-P is found in cell walls of higher plants and some green algae<ref>PMID:11904225</ref>. |
== Structural highlights == | == Structural highlights == | ||
| - | The biological assembly of Metallo Kdo-8-phosphate synthase from ''Neisseria meningitidis'' is <scene name='52/525184/Cv/ | + | The biological assembly of Metallo Kdo-8-phosphate synthase from ''Neisseria meningitidis'' is <scene name='52/525184/Cv/7'>homotetramer</scene>. The <scene name='52/525184/Cv/8'>active site of the metallo KPS contains a divalent cation and the PEP substrate</scene><ref>PMID:19447118</ref>. Water molecules shown as red spheres. |
</StructureSection> | </StructureSection> | ||
==3D structures of Kdo-8-phosphate synthase== | ==3D structures of Kdo-8-phosphate synthase== | ||
| Line 61: | Line 61: | ||
*Non-metallo Kdo8P | *Non-metallo Kdo8P | ||
| - | **[[1d9e]], [[1gg0]], [[1x8f]] – EcKPS - ''Escherichia coli''<br /> | + | **[[1d9e]], [[1gg0]], [[1x8f]], [[6u57]] – EcKPS - ''Escherichia coli''<br /> |
**[[1o60]] - KPS – ''Haemophilus influenza''<br /> | **[[1o60]] - KPS – ''Haemophilus influenza''<br /> | ||
**[[3e9a]] - KPS – ''Vibrio cholerae''<br /> | **[[3e9a]] - KPS – ''Vibrio cholerae''<br /> | ||
**[[3fs2]] - KPS – ''Brucella melitensis''<br /> | **[[3fs2]] - KPS – ''Brucella melitensis''<br /> | ||
| - | **[[3t4c]], [[3tml]] - KPS – ''Burkholderia ambifaria'' | + | **[[3t4c]], [[3tml]] - KPS – ''Burkholderia ambifaria''<br /> |
| + | **[[6mdy]] – KPS – ''Legionella pneumophila''<br /> | ||
*''Non-metallo Kdo8P binary complex'' | *''Non-metallo Kdo8P binary complex'' | ||
Current revision
| |||||||||||
3D structures of Kdo-8-phosphate synthase
Updated on 11-October-2021
References
- ↑ Krosky DJ, Alm R, Berg M, Carmel G, Tummino PJ, Xu B, Yang W. Helicobacter pylori 3-deoxy-D-manno-octulosonate-8-phosphate (KDO-8-P) synthase is a zinc-metalloenzyme. Biochim Biophys Acta. 2002 Feb 11;1594(2):297-306. PMID:11904225
- ↑ Cochrane FC, Cookson TV, Jameson GB, Parker EJ. Reversing evolution: re-establishing obligate metal ion dependence in a metal-independent KDO8P synthase. J Mol Biol. 2009 Jul 24;390(4):646-61. Epub 2009 May 15. PMID:19447118 doi:10.1016/j.jmb.2009.05.014