User:Karsten Theis/overall views
From Proteopedia
| (45 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | __NOTOC__ | ||
==Introduction== | ==Introduction== | ||
| - | This is a collection of how protein structures are depicted in publications. The most common views show | + | <gallery> |
| - | * domains | + | Image:UvrB fold.JPG |
| - | + | Image:UvrB charge.JPG | |
| + | Image:UvrB slab core.JPG | ||
| + | Image:UvrB consurf.JPG | ||
| + | </gallery> | ||
| + | This is a collection of how entire protein structures are depicted in publications. The most common views show | ||
| + | * fold of domains | ||
* charge distribution | * charge distribution | ||
| - | * | + | * hydrophobic patches |
| + | * surface conservation | ||
| + | * superpositions with related structures | ||
==Standard and other views== | ==Standard and other views== | ||
| - | In publications where figures are two dimensional and non-interactive, researchers have to choose a view that shows as much of the interesting features of the protein as possible. Often, when that is not possible, there will be two orthoganal views (e.g. the second rotated by 90 or 180 degrees. The protein used as an example here is the DNA repair enzyme UvrB in complex with ATP (PDB ID 1d9z). This protein not only binds to ATP, but also to DNA and to another DNA repair protein, UvrA. As you look at the various ways protein structures are depicted, you can zoom in to the different binding surfaces or zoom out to the standard view showing the entire protein with the "business" side facing you. | + | In publications where figures are two dimensional and non-interactive, researchers have to choose a view that shows as much of the interesting features of the protein as possible. Often, when that is not possible, there will be two orthoganal views (e.g. the second rotated by 90 or 180 degrees. The protein used as an example here is the DNA repair enzyme UvrB in complex with ATP (PDB ID 1d9z)<ref>PMID:10601012</ref>. This protein not only binds to ATP, but also to DNA and to another DNA repair protein, UvrA. As you look at the various ways protein structures are depicted, you can zoom in to the different binding surfaces or zoom out to the standard view showing the entire protein with the "business" side facing you. |
<jmol> | <jmol> | ||
<jmolButton> | <jmolButton> | ||
| Line 33: | Line 41: | ||
</jmolButton> | </jmolButton> | ||
</jmol> | </jmol> | ||
| + | |||
<jmol> | <jmol> | ||
| Line 41: | Line 50: | ||
</jmolButton> | </jmolButton> | ||
</jmol> | </jmol> | ||
| + | |||
<jmol> | <jmol> | ||
| Line 65: | Line 75: | ||
==Types of overall views== | ==Types of overall views== | ||
| - | <StructureSection load='' size='340' side='right' caption=' | + | <StructureSection load='1d9z' size='340' side='right' caption='Automatically generated figure for UvrB structure, PDBID 1d9z' scene=''> |
| - | === | + | ===Ribbon diagram showing fold=== |
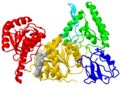
| - | The first view of a protein shown in a publication is often a cartoon of the <scene name='78/780454/Domains/7'> | + | [[Image:UvrB fold.JPG|none|thumb|200px]] |
| + | |||
| + | |||
| + | The first view of a protein shown in a publication is often a cartoon of the <scene name='78/780454/Domains/7'>fold colored by domains</scene>. The fold of a protein refers to how secondary structure elements are assembled in three dimensions, and it is often shown in a cartoon (Richardson diagram). | ||
<jmol> | <jmol> | ||
| Line 131: | Line 144: | ||
===Surface charges=== | ===Surface charges=== | ||
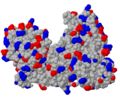
| + | [[Image:UvrB charge.JPG|none|thumb|200px]] | ||
| + | |||
| + | |||
To show where negatively or positively charged molecules are bound, 2D-figures sometimes show surfaces colored by an electrostatic potential calculated from the point charges on Asp, Glu, Arg, Lys and - if the charge state is known - His. The common color scheme is blue for positive and red for negative potential (corresponding nicely to the CPK color scheme with blue nitrogen atoms - carrying a positive formal charge - and red oxygen atoms - carrying a negative formal charge). A quick and simple approximation in Jmol is to show the molecule as spacefill, and <scene name='78/780454/Charges/1'>color the charged side chains</scene>. (You could also just color the side chain oxygen and nitrogen atoms, but you then ignore charges of disordered atoms missing in the model but present in the protein.) The UvrB protein shown does not exhibit any obvious regions of positive or negative charges. | To show where negatively or positively charged molecules are bound, 2D-figures sometimes show surfaces colored by an electrostatic potential calculated from the point charges on Asp, Glu, Arg, Lys and - if the charge state is known - His. The common color scheme is blue for positive and red for negative potential (corresponding nicely to the CPK color scheme with blue nitrogen atoms - carrying a positive formal charge - and red oxygen atoms - carrying a negative formal charge). A quick and simple approximation in Jmol is to show the molecule as spacefill, and <scene name='78/780454/Charges/1'>color the charged side chains</scene>. (You could also just color the side chain oxygen and nitrogen atoms, but you then ignore charges of disordered atoms missing in the model but present in the protein.) The UvrB protein shown does not exhibit any obvious regions of positive or negative charges. | ||
===Hydrophobic side chains=== | ===Hydrophobic side chains=== | ||
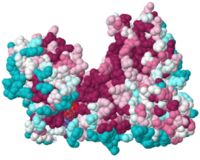
| + | [[Image:UvrB slab core.JPG|none|thumb|200px]] | ||
| + | |||
| + | |||
To show <scene name='78/780454/Hydrophobic/3'>hydrophobic patches</scene> on the surface of the protein, we can color the carbons on the side chains of Met, Ile, Leu, Val, Phe, Tyr and Trp gray while all other side chain atoms are the color orchid and the backbone is white (the OH group of Tyr is also shown in purple. | To show <scene name='78/780454/Hydrophobic/3'>hydrophobic patches</scene> on the surface of the protein, we can color the carbons on the side chains of Met, Ile, Leu, Val, Phe, Tyr and Trp gray while all other side chain atoms are the color orchid and the backbone is white (the OH group of Tyr is also shown in purple. | ||
| Line 171: | Line 190: | ||
*Is there one hydrophobic core or multiple? | *Is there one hydrophobic core or multiple? | ||
*How are domains related to hydrophobic cores (you might have to go back to the first figure)? | *How are domains related to hydrophobic cores (you might have to go back to the first figure)? | ||
| - | *Does domain 2 behave differently than the others (it is the most disordered domain, is missing several sidechains and its connectivity was updated in later models where the domain was more ordered, e.g. PDBID [[1t5l] and [[2nmv]]. | + | *Does domain 2 behave differently than the others (it is the most disordered domain, is missing several sidechains and its connectivity was updated in later models where the domain was more ordered, e.g. PDBID [[1t5l]] and [[2nmv]]. |
*Where are the OH-groups of tyrosines (shown in orchid)? | *Where are the OH-groups of tyrosines (shown in orchid)? | ||
*What would happen if we place this molecule into a nonpolar solvent such as cyclohexane? | *What would happen if we place this molecule into a nonpolar solvent such as cyclohexane? | ||
| Line 204: | Line 223: | ||
===Degree of conservation=== | ===Degree of conservation=== | ||
| + | [[Image:UvrB consurf.JPG|none|thumb|200px]] | ||
| + | |||
| + | |||
To show evolutionary <jmol> | To show evolutionary <jmol> | ||
| Line 209: | Line 231: | ||
<script> | <script> | ||
select protein; define ~consurf_to_do selected | select protein; define ~consurf_to_do selected | ||
| - | consurf_initial_scene = true; script "/wiki/ConSurf/d9/1d9z_consurf.spt" | + | consurf_initial_scene = true; script "/wiki/ConSurf/d9/1d9z_consurf.spt";select protein;spacefill only |
</script> | </script> | ||
<text>conservation</text> | <text>conservation</text> | ||
</jmolLink> | </jmolLink> | ||
| - | </jmol>, we use data from [[Introduction to Evolutionary Conservation|ConSurf]]. | + | </jmol>, we use data from [[Introduction to Evolutionary Conservation|ConSurf]] (scene <scene name='78/780454/Conservation/1'>oriented as 2D figure</scene> on the left). |
| + | |||
| + | <jmol> | ||
| + | <jmolRadioGroup> | ||
| + | <item> | ||
| + | <script>hide (hidden and not protein) or (protein and not (1-90, 117-156, 245-414))</script> | ||
| + | <text>domain 1(a+b)</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>hide (hidden and not protein) or (protein and not (157-244))</script> | ||
| + | <text>domain 2</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>hide (hidden and not protein) or (protein and not (415-600))</script> | ||
| + | <text>domain 3</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>hide (hidden and not protein) or (protein and not 91-116)</script> | ||
| + | <text>hairpin</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>display displayed or protein</script> | ||
| + | <text>all domains</text> | ||
| + | <checked>true</checked> | ||
| + | |||
| + | </item> | ||
| + | |||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| + | |||
| + | <jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>display displayed or ATP</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>hide ATP or hidden</scriptWhenUnchecked> | ||
| + | <checked>true</checked> | ||
| + | <text>ATP molecule</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol><jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>display displayed or MG</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>hide MG or hidden</scriptWhenUnchecked> | ||
| + | <checked>true</checked> | ||
| + | <text>Mg(2+) ion</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol> | ||
| + | |||
| + | |||
Looking at the 3D scene, and using the view buttons and the buttons to turns things on and off, you can answer questions like: | Looking at the 3D scene, and using the view buttons and the buttons to turns things on and off, you can answer questions like: | ||
| Line 221: | Line 289: | ||
* Which domains might be part of a large family of related structures, and which might be more unique? | * Which domains might be part of a large family of related structures, and which might be more unique? | ||
* Are the cysteine residues conserved in this protein, which does not form disulfide bonds (use the command "display CYS" in jmol after you open the jmol console by right clicking on the 3D viewer)? | * Are the cysteine residues conserved in this protein, which does not form disulfide bonds (use the command "display CYS" in jmol after you open the jmol console by right clicking on the 3D viewer)? | ||
| + | |||
| + | The original figures of the primary citation used a trick to see better into the active site between domains 1a and 3: domain 3 was rotated outwards to open up the view. You can use the control below to achieve a similar effect: | ||
| + | |||
| + | <jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>select 415-600;rotateselected {558.CA}{580.CA} 120 </scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>select 415-600;rotateselected {558.CA}{580.CA} -120</scriptWhenUnchecked> | ||
| + | <checked>false</checked> | ||
| + | <text>butterflied molecule</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol> | ||
| + | |||
| + | |||
<jmol> | <jmol> | ||
| Line 238: | Line 319: | ||
===Backbone trace and Superpositions=== | ===Backbone trace and Superpositions=== | ||
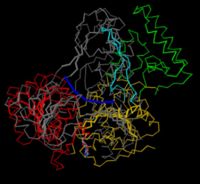
| + | [[Image:UvrB superposition.JPG|none|thumb|200px]] | ||
| + | |||
| + | |||
Superpositions of multiple structures is used to show similarity in their folding. It is very difficult to make a clear picture of two entire proteins on top of each other, and the traditional way of decluttering is to use a <jmol> | Superpositions of multiple structures is used to show similarity in their folding. It is very difficult to make a clear picture of two entire proteins on top of each other, and the traditional way of decluttering is to use a <jmol> | ||
<jmolLink> | <jmolLink> | ||
| Line 247: | Line 331: | ||
</jmol>. This is also a great type of figure if you want to measure distances between alpha carbons, or investigate the overall structure in other ways by clicking on residues. | </jmol>. This is also a great type of figure if you want to measure distances between alpha carbons, or investigate the overall structure in other ways by clicking on residues. | ||
| - | The following radio buttons allow you to explore the overall structure: | + | The following radio buttons allow you to explore the overall structure by clicking on one atom or on a pair of atoms (the last two will change the structure itself, not only how it is represented): |
<jmol> | <jmol> | ||
<jmolRadioGroup> | <jmolRadioGroup> | ||
| + | <item> | ||
| + | <script>set picking off</script> | ||
| + | <text>doubleclick to measure (default)</text> | ||
| + | <checked>true</checked> | ||
| + | </item> | ||
<item> | <item> | ||
<script>set picking center</script> | <script>set picking center</script> | ||
| Line 257: | Line 346: | ||
<script>set picking label</script> | <script>set picking label</script> | ||
<text>label atom</text> | <text>label atom</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>console; set picking MEASURE SEQUENCE</script> | ||
| + | <text>show sequence</text> | ||
</item> | </item> | ||
<item> | <item> | ||
<script>set picking CONNECT</script> | <script>set picking CONNECT</script> | ||
<text>new bond (woah!)</text> | <text>new bond (woah!)</text> | ||
| - | <checked>true</checked> | ||
</item> | </item> | ||
<item> | <item> | ||
<script>set picking DRAGLIGAND</script> | <script>set picking DRAGLIGAND</script> | ||
| - | <text>drag | + | <text>drag Mg-ion (what?!)</text> |
| + | </item> | ||
| + | |||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| + | |||
| + | Some choices need the Jmol console, which will open somewhere automatically. | ||
| + | |||
| + | See if you can answer the following questions: | ||
| + | *What are the residues in domain 1 near the ATP ligand? Label them. | ||
| + | *ATP is bound through a conserved structural element called the P-loop, which contain "GKT" as a sequence motif. Using the "show sequence" radio button, figure out the residue numbers of this motif. | ||
| + | * What are the dimensions of domain 3 (first, center on an atom in the middle of domain 3 and zoom in. Then, do some distance measurements)? | ||
| + | * Use the Mg-ion as a marker to show the tunnel formed by domain 1b (green) and the beta hairpin structure (cyan). | ||
| + | * Make a new connection between any two atoms. If you don't like the new bond, click the two atoms again to remove it. | ||
| + | |||
| + | To superimpose UvrB with the related helicase NS3 from the hepatitic C virus, you have to find pairs of corresponding residues (e.g. K45 of UvrB and K210 from NS3 are equivalent residues of the P-loop) and minimize the distance between pairs. The resulting <scene name='78/780454/Superposition/4'>superposition</scene> shows that while some domains (which?) are similar, others are unique to one or the other. | ||
| + | |||
| + | <jmol> | ||
| + | <jmolRadioGroup> | ||
| + | <item> | ||
<checked>true</checked> | <checked>true</checked> | ||
| + | <script>model 1</script> | ||
| + | <text>UvrB only</text> | ||
</item> | </item> | ||
| + | <item> | ||
| + | <script>model 2</script> | ||
| + | <text>NS3 only</text> | ||
| + | </item> | ||
| + | <item> | ||
| + | <script>model 0</script> | ||
| + | <text>both</text> | ||
| + | </item> | ||
| + | </jmolRadioGroup> | ||
| + | </jmol> | ||
| + | |||
| + | |||
| + | This superposition is complicated. Try hiding some of the elements to see clearer, and open a popup 3D browser to view a larger version. Also, rotate the molecules a bit to see them from different angles. You can use the wobble button at the bottom of the page as well. In the original publication [http://emboj.embopress.org/content/18/24/6899.figures-only](Fig. 4), the figure is shown in stereo for better viewing, and domain 2 is omitted. In the Jmol browser used here, you can turn on stereo as well. This is best done in the pop-up window using the right-click menu, but depending on your eyes, you might need stereo glasses to experience the effect. | ||
| + | |||
| + | <jmol> | ||
| + | <jmolRadioGroup> | ||
<item> | <item> | ||
| - | <script> | + | <script>ns3_sheet = {1.2 and (198-203, 225-229, 251-253, 258-259, 265-269, 286-290, 317-322, 336-340, 347-349, 388-391, 406-410, 424-427, 430-437, 445-452, 471-475, 609-610)} |
| - | <text> | + | ; hide protein and not (ns3_sheet or (1.1 and sheet))</script> |
| + | <text>strands only</text> | ||
| + | </item> | ||
| + | <item> | ||
<checked>true</checked> | <checked>true</checked> | ||
| + | <script>display displayed or protein</script> | ||
| + | <text>everything</text> | ||
</item> | </item> | ||
</jmolRadioGroup> | </jmolRadioGroup> | ||
Current revision
Introduction
This is a collection of how entire protein structures are depicted in publications. The most common views show
- fold of domains
- charge distribution
- hydrophobic patches
- surface conservation
- superpositions with related structures
Standard and other views
In publications where figures are two dimensional and non-interactive, researchers have to choose a view that shows as much of the interesting features of the protein as possible. Often, when that is not possible, there will be two orthoganal views (e.g. the second rotated by 90 or 180 degrees. The protein used as an example here is the DNA repair enzyme UvrB in complex with ATP (PDB ID 1d9z)[1]. This protein not only binds to ATP, but also to DNA and to another DNA repair protein, UvrA. As you look at the various ways protein structures are depicted, you can zoom in to the different binding surfaces or zoom out to the standard view showing the entire protein with the "business" side facing you.
Types of overall views
| |||||||||||
References
- ↑ Theis K, Chen PJ, Skorvaga M, Van Houten B, Kisker C. Crystal structure of UvrB, a DNA helicase adapted for nucleotide excision repair. EMBO J. 1999 Dec 15;18(24):6899-907. PMID:10601012 doi:10.1093/emboj/18.24.6899