Retroviral Integrase
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
==Function== | ==Function== | ||
| - | [[Retroviral Integrase]] is an essential retroviral enzyme that binds to viral DNA and inserts it into a host cell chromosome. The reverse transcribed cDNA of human immunodeficiency virus type 1 (HIV-1) is inserted in the host cell genome in order increase pathogen fitness and virulence. Integrase is produced by a class of retrovirus (like HIV) and is used by the virus to incorporate its genetic material into the host cell DNA. The host cellular machinery then produces mRNA and then protein from the incorporated genetic material, thus replicating the virus. Although several integrase inhibiting drugs have been investigated, the mechanism responsible for strand-transfer inhibition action remains to be elucidated <ref>PMID:19915684</ref>. However, Hare el al (2010)<ref name=Hare_2010>PMID:20118915</ref> determined the structural constituents of retroviral integration. Further elucidation of the complete structure of the retroviral integrase, and its application to regulate functional and enzymatic activities could potentially enable researchers to delay the progression of retroviral diseases. Moreover, study of HIV-1 integration could lead to a promising new target, and contribute to the generation pharmacophore models for antiviral therapy<ref>deJesus, Edwin [http://www.thebody.com/content/art1352.html#ii HIV Antiretroviral Agents in Development]. The Body: The Complete HIV/AIDS Resource. March 30, 2006</ref>. <br/> | + | [[Retroviral Integrase]] is an essential retroviral enzyme that binds to viral DNA and inserts it into a host cell chromosome. The complex of integrase and DNA is called '''intasome.''' The reverse transcribed cDNA of human immunodeficiency virus type 1 (HIV-1) is inserted in the host cell genome in order increase pathogen fitness and virulence. Integrase is produced by a class of retrovirus (like HIV) and is used by the virus to incorporate its genetic material into the host cell DNA. The host cellular machinery then produces mRNA and then protein from the incorporated genetic material, thus replicating the virus. Although several integrase inhibiting drugs have been investigated, the mechanism responsible for strand-transfer inhibition action remains to be elucidated <ref>PMID:19915684</ref>. However, Hare el al (2010)<ref name=Hare_2010>PMID:20118915</ref> determined the structural constituents of retroviral integration. Further elucidation of the complete structure of the retroviral integrase, and its application to regulate functional and enzymatic activities could potentially enable researchers to delay the progression of retroviral diseases. Moreover, study of HIV-1 integration could lead to a promising new target, and contribute to the generation pharmacophore models for antiviral therapy<ref>deJesus, Edwin [http://www.thebody.com/content/art1352.html#ii HIV Antiretroviral Agents in Development]. The Body: The Complete HIV/AIDS Resource. March 30, 2006</ref>. <br/> |
HIV Integrase inhibitors: [http://www.isentress.com Raltegravir], marketed as Isentress is currently approved as a therapeutic inhibitor of HIV integrase. It was approved on October 12, 2007. | HIV Integrase inhibitors: [http://www.isentress.com Raltegravir], marketed as Isentress is currently approved as a therapeutic inhibitor of HIV integrase. It was approved on October 12, 2007. | ||
[See below for a table of antiretroviral drugs with trade name, company, patents, and notes.] | [See below for a table of antiretroviral drugs with trade name, company, patents, and notes.] | ||
| Line 19: | Line 19: | ||
[[Image:HIV_Stats.jpg|thumb|alt=Alt text|In 2010, there are more than 25 million people have died of AIDS, and it is estimated that approximately 33 million people are living with HIV.]] | [[Image:HIV_Stats.jpg|thumb|alt=Alt text|In 2010, there are more than 25 million people have died of AIDS, and it is estimated that approximately 33 million people are living with HIV.]] | ||
| - | To date, more than 25 million people have died of AIDS and it is estimated that approximately 33 million people are living with HIV today<ref> [https://aidsinfo.nih.gov AIDS-Info]</ref>. For retroviral integrase inhibitors see [[Raltegravir]] and [[Retroviral Integrase Inhibitor Pharmacokinetics]]. | + | To date, more than 25 million people have died of AIDS and it is estimated that approximately 33 million people are living with HIV today<ref> [https://aidsinfo.nih.gov AIDS-Info]</ref>. For retroviral integrase inhibitors see [[Raltegravir]], [[Genvoya]] and [[Retroviral Integrase Inhibitor Pharmacokinetics]]. |
==Impact of Structure== | ==Impact of Structure== | ||
| Line 25: | Line 25: | ||
Three-dimensional structures for certain host-cell proteins critical to understanding the mechanism of HIV infection and virulence have emerged from X-ray crystallographic analyses. HIV protease and integrase structures are among the highest ranked structures that have contributed to saving many lives and added to the quality of life of many HIV-afflicted individuals. It is implemented in [[Structure-based drug design|structure-based drug design]] to develop [http://en.wikipedia.org/wiki/Protease_inhibitors protease inhibitors] and [http://en.wikipedia.org/wiki/Integrase_inhibitors integrase inhibitors], and is used as a significant component of ''highly active anti-retroviral therapy'' ([http://en.wikipedia.org/wiki/Haart HAART]). | Three-dimensional structures for certain host-cell proteins critical to understanding the mechanism of HIV infection and virulence have emerged from X-ray crystallographic analyses. HIV protease and integrase structures are among the highest ranked structures that have contributed to saving many lives and added to the quality of life of many HIV-afflicted individuals. It is implemented in [[Structure-based drug design|structure-based drug design]] to develop [http://en.wikipedia.org/wiki/Protease_inhibitors protease inhibitors] and [http://en.wikipedia.org/wiki/Integrase_inhibitors integrase inhibitors], and is used as a significant component of ''highly active anti-retroviral therapy'' ([http://en.wikipedia.org/wiki/Haart HAART]). | ||
| - | While existing antiretroviral agents improve the quality of life as well as extending the life of many patients, it fails to eradicate the disease. Studies in integrase inhibitors show that combination with other antiretroviral drugs diminish viral adaptations, and may have the potential to be used for ''salvage therapy'' for patients who have acquired resistance to other drugs. For more, please see [[User:Eric_Martz/Molecular_Playground/HIVDrug|AIDS Before Protease Inhibitors & HIV Protease Inhibitors: A Breakthrough]]. | + | While existing antiretroviral agents improve the quality of life as well as extending the life of many patients, it fails to eradicate the disease. Studies in integrase inhibitors show that combination with other antiretroviral drugs diminish viral adaptations, and may have the potential to be used for ''salvage therapy'' for patients who have acquired resistance to other drugs. For more, please see<br /> |
| + | *[[User:Eric_Martz/Molecular_Playground/HIVDrug|AIDS Before Protease Inhibitors & HIV Protease Inhibitors: A Breakthrough]]<br /> | ||
| + | *[[Treatments:Retroviral Integrase Inhibitor Pharmacokinetics References]]. | ||
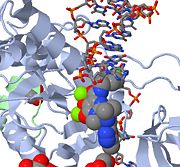
==PFV Intasome Crystallization== | ==PFV Intasome Crystallization== | ||
| Line 60: | Line 62: | ||
*<scene name='38/386179/Cv/3'>Zinc coordination site</scene>. | *<scene name='38/386179/Cv/3'>Zinc coordination site</scene>. | ||
*<scene name='38/386179/Cv/4'>K coordination site</scene>. | *<scene name='38/386179/Cv/4'>K coordination site</scene>. | ||
| - | </StructureSection> | ||
| - | __NOTOC__ | ||
==Integrase Inhibitors== | ==Integrase Inhibitors== | ||
| Line 83: | Line 83: | ||
<br /> | <br /> | ||
| - | == 3D | + | ==3D structures of retroviral integrase== |
| - | + | [[Retroviral integrase 3D structures]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | </StructureSection> | ||
==References== | ==References== | ||
Current revision
| |||||||||||
References
- ↑ Pandey KK, Grandgenett DP. HIV-1 Integrase Strand Transfer Inhibitors: Novel Insights into their Mechanism of Action. Retrovirology (Auckl). 2008 Nov 5;2:11-16. PMID:19915684
- ↑ 2.0 2.1 2.2 2.3 Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P. Retroviral intasome assembly and inhibition of DNA strand transfer. Nature. 2010 Mar 11;464(7286):232-6. Epub 2010 Jan 31. PMID:20118915 doi:10.1038/nature08784
- ↑ deJesus, Edwin HIV Antiretroviral Agents in Development. The Body: The Complete HIV/AIDS Resource. March 30, 2006
- ↑ Braun, J.F., Cronje, R.J and Henderson, M.G. HIV-1 Integrase Inhibitors Inhibitors (2008). www.prn.org Volume 13, Pages 1–9
- ↑ AIDS-Info
- ↑ Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, Breidinger SA, Mangin EC, Azrolan N, Greenberg HE, Haazen W, Stone JA, Gottesdiener KM, Wagner JA. Minimal effects of ritonavir and efavirenz on the pharmacokinetics of raltegravir. Antimicrob Agents Chemother. 2008 Dec;52(12):4338-43. Epub 2008 Oct 6. PMID:18838589 doi:10.1128/AAC.01543-07
Further Reading
- GEN News Highlights "Scientists Solve 3-D Crystal Structure of Retroviral Integrase Bound to Viral DNA", Genetic Engineering & Biotechnology News February 1, 2010.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Rhysly Martinez, Joel L. Sussman, Alexander Berchansky, David Canner, Jordan Heard, Eugene Babcock, Garrett Asanuma