We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Reverse transcriptase
From Proteopedia
(Difference between revisions)
| (7 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
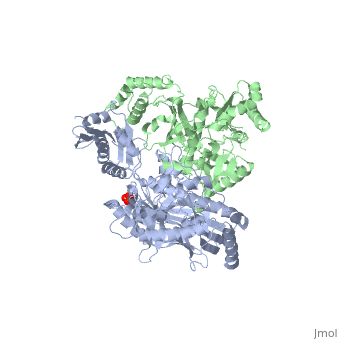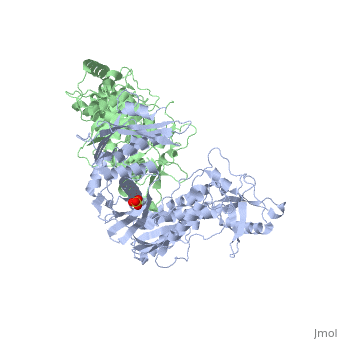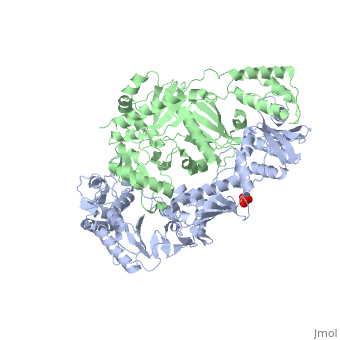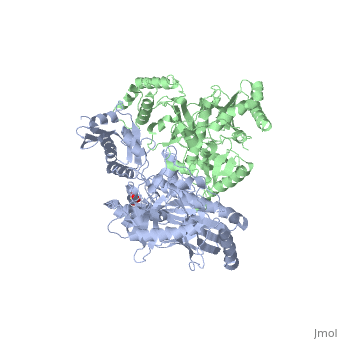
| - | [[Reverse transcriptase]] (RT) or '''RNA-dependent DNA polymerase''' transcribes single-stranded RNA into double-stranded [[DNA]]. HIV-1 RT is from the human immunodeficiency virus and is a heterodimer of P66 and P51 subchains. P15 is its RNAse H domain. There are two types of inhibitors for RT: NNRTIs are the non-nucleoside inhibitors, and NRTIs are the | + | [[Reverse transcriptase]] (RT) or '''RNA-dependent DNA polymerase''' transcribes single-stranded RNA into double-stranded [[DNA]]. HIV-1 RT is from the human immunodeficiency virus and is a heterodimer of P66 and P51 subchains. P15 is its RNAse H domain. There are two types of inhibitors for RT: '''NNRTIs''' are the non-nucleoside inhibitors, and '''NRTIs''' are the nucleoside inhibitors. Being the protein that gives their name to Retroviruses, Reverse Transcriptase is, along with [[Hiv protease|Protease]] and Integrase, the most important part of the protein system involved in the process of infection and reproduction for viruses like HIV, MuLV and AMV. RT has the unusual property of transcribing ssRNA into dsDNA going against the Central Dogma of Molecular Biology. |
Since its discovery in 1970, the study of its properties and mechanisms of action have been of high interest among the scientific community due to the unique properties that makes it an important medical target enzyme and important tool for genetic engineering applications like RT-PCR in the construction of cDNA libraries. See also <br /> | Since its discovery in 1970, the study of its properties and mechanisms of action have been of high interest among the scientific community due to the unique properties that makes it an important medical target enzyme and important tool for genetic engineering applications like RT-PCR in the construction of cDNA libraries. See also <br /> | ||
*[[Transcription and RNA Processing]]<br /> | *[[Transcription and RNA Processing]]<br /> | ||
*[[HIV-1 Reverse Transcriptase in Complex with Nevirapine]]<br /> | *[[HIV-1 Reverse Transcriptase in Complex with Nevirapine]]<br /> | ||
| + | *[[Efavirenz]]<br /> | ||
| + | *[[Emtricitabine]]<br /> | ||
| + | *[[Efavirenz/emtricitabine/tenofovir]]<br /> | ||
*[[Phl p 2]]<br /> | *[[Phl p 2]]<br /> | ||
| + | *[[Tenofovir disoproxil]]<br /> | ||
*[[AZT-resistant HIV-1 reverse transcriptase]]<br /> | *[[AZT-resistant HIV-1 reverse transcriptase]]<br /> | ||
*[[Catalytic Subunit of T. Castaneum TERT Polymerase]].<br /> | *[[Catalytic Subunit of T. Castaneum TERT Polymerase]].<br /> | ||
| Line 12: | Line 16: | ||
*[[Reverse Transcriptase (Hebrew)]]<br /> | *[[Reverse Transcriptase (Hebrew)]]<br /> | ||
| - | Reverse Transcriptase is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. <scene name='Reverse_transcriptase/Presentation/3' caption='The hand-like two-enzymes-in-one protein that amazingly makes DNA from RNA'>-- CBI Molecular Playground Model --</scene> | + | Reverse Transcriptase is one of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst (see [[UMass Chem 423 Student Projects 2011-2#HIV Reverse Transcriptase|HIV Reverse Transcriptase (UMass Chem 423 Student Projects 2011-2)]]) and on display at the [http://www.molecularplayground.org/ Molecular Playground]. <scene name='Reverse_transcriptase/Presentation/3' caption='The hand-like two-enzymes-in-one protein that amazingly makes DNA from RNA'>-- CBI Molecular Playground Model --</scene> |
{{Clear}} | {{Clear}} | ||
| Line 32: | Line 36: | ||
One of the principal issues about this protein compared to usual DNA polymerase (besides to the similarity with the Klenow fragment), is the lack of a correction mechanism (usually made by DNA PolIII in the [[User:Karl E. Zahn/RB69 DNA polymerase (GP43)|DNA Polymerase]]); this deficiency increases the number of errors, producing more mutations and therefore giving more facultative and resistance ability to the virus. | One of the principal issues about this protein compared to usual DNA polymerase (besides to the similarity with the Klenow fragment), is the lack of a correction mechanism (usually made by DNA PolIII in the [[User:Karl E. Zahn/RB69 DNA polymerase (GP43)|DNA Polymerase]]); this deficiency increases the number of errors, producing more mutations and therefore giving more facultative and resistance ability to the virus. | ||
{{Clear}} | {{Clear}} | ||
| - | + | ||
== 3D Structures of Reverse transcriptase == | == 3D Structures of Reverse transcriptase == | ||
| + | [[Reverse transcriptase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | }} | ||
==See Also== | ==See Also== | ||
Current revision
| |||||||||||
See Also
- Reverse Transcriptase at Wikipedia
- Molecule of the Month (09/2002) at RCSB Protein Data Bank
- List of Reverse Transcriptase articles at Proteopedia and at RCSB Protein Data Bank
- Model of Reverse Transcriptase as one of the CBI Molecules on the Molecular Playground
- See Transcription for additional Proteopedia articles on the subject.
- For additional information, see: Human Immunodeficiency Virus
- For additional information, see: Transcription and RNA Processing
References
- ↑ Kohlstaedt LA, Wang J, Friedman JM, Rice PA, Steitz TA. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992 Jun 26;256(5065):1783-90. PMID:1377403 doi:[http://dx.doi.org/10.1126/science.1377403 http://dx.doi.org/10.1126/science.1377403
- ↑ Sarafianos SG, Marchand B, Das K, Himmel DM, Parniak MA, Hughes SH, Arnold E. Structure and function of HIV-1 reverse transcriptase: molecular mechanisms of polymerization and inhibition. J Mol Biol. 2009 Jan 23;385(3):693-713. doi: 10.1016/j.jmb.2008.10.071. Epub 2008, Nov 3. PMID:19022262 doi:http://dx.doi.org/10.1016/j.jmb.2008.10.071
- ↑ ConSurf: Using Evolutionary Data to Raise Testable Hypotheses about Protein Function DOI: 10.1002/ijch.201200096
- ↑ Abbondanzieri EA, Bokinsky G, Rausch JW, Zhang JX, Le Grice SF, Zhuang X. Dynamic binding orientations direct activity of HIV reverse transcriptase. Nature. 2008 May 8;453(7192):184-9. PMID:18464735 doi:10.1038/nature06941
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Daniel Moyano-Marino, Joel L. Sussman, Alexander Berchansky, David Canner, Amol Kapoor, Jaime Prilusky, Brian Foley, Lynmarie K Thompson, Eric Martz