Uridine 5'-monophosphate synthase
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='' size='350' side='right' caption='Human uridine 5-monophosphate synthase OPD subunit dimer complex with UMP [[2qcd]]' scene='52/526172/Cv/1' pspeed='8'> | <StructureSection load='' size='350' side='right' caption='Human uridine 5-monophosphate synthase OPD subunit dimer complex with UMP [[2qcd]]' scene='52/526172/Cv/1' pspeed='8'> | ||
== Function == | == Function == | ||
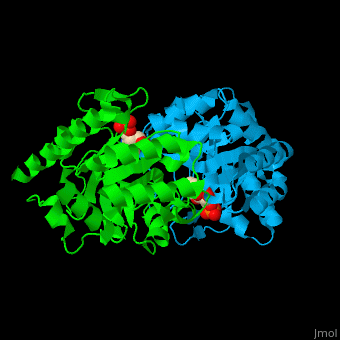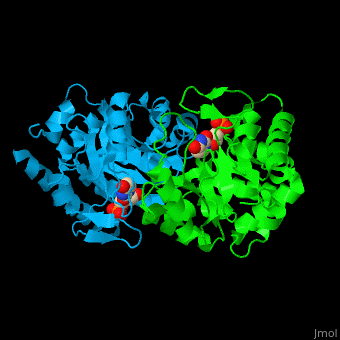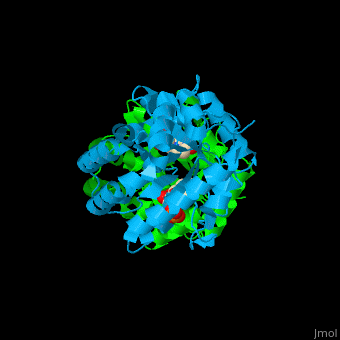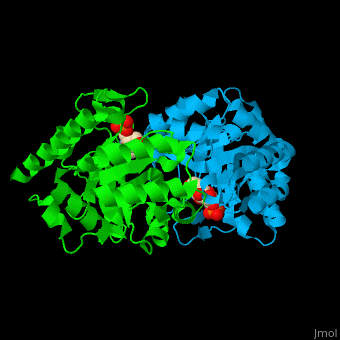
| - | '''Uridine 5’-monophosphate synthase''' (UMPS) is a bifunctional enzyme catalyzes the formation of uridine monophosphate (UMP)<ref>PMID:8631878</ref>. UMPS N-terminal domain is an '''orotate phosphoribosyltransferase''' (OPRT) subunit which catalyzes the addition of ribose-phosphate to orotate forming orotidine 5’-monophosphate (OMP). The C-terminal subunit is '''orotidine 5’-phosphate decarboxylase''' (OPD) or '''OMP decarboxylase''' which decarboxylates OMP to form UMP. Potent inhibitors of OPD are BMP – a barbituric acid derivative and xanthosine-5'-monophosphate (XMP). | + | '''Uridine 5’-monophosphate synthase''' (UMPS) is a bifunctional enzyme which catalyzes the formation of uridine monophosphate (UMP)<ref>PMID:8631878</ref>. UMPS '''N-terminal domain''' is an '''orotate phosphoribosyltransferase''' (OPRT) subunit which catalyzes the addition of ribose-phosphate to orotate forming orotidine 5’-monophosphate (OMP). The '''C-terminal subunit''' is '''orotidine 5’-phosphate decarboxylase''' (OPD) or '''OMP decarboxylase''' which decarboxylates OMP to form UMP. Potent inhibitors of OPD are BMP – a barbituric acid derivative and xanthosine-5'-monophosphate (XMP). |
== Disease == | == Disease == | ||
| Line 7: | Line 7: | ||
== Structural highlights == | == Structural highlights == | ||
| - | <scene name='52/526172/Cv/ | + | <scene name='52/526172/Cv/8'>UMPS contains UMP</scene> in its <scene name='52/526172/Cv/9'>nucleotide-binding pocket</scene><ref>PMID:18184586</ref> (the surface of chain B doesn't shown). Water molecules are shown as red spheres. |
| + | ==3D structures of uridine 5'-monophosphate synthase== | ||
| + | [[Uridine 5'-monophosphate synthase 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
| - | ==3D structures of uridine 5'-monophosphate synthase== | ||
| - | |||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | |||
| - | *Uridine 5’-monophosphate synthase OMP decarboxylase subunit | ||
| - | |||
| - | **[[3gdk]] - yOPD – yeast<br /> | ||
| - | **[[1dqw]], [[3gdr]], [[3gdm]] – yOPD (mutant) <br /> | ||
| - | **[[2eaw]], [[2jgy]], [[2p1f]], [[2qcc]], [[2qce]] - hOPD – human<br /> | ||
| - | **[[4n2y]] – AfOPD – ''Archaeoglobus fulgidus''<br /> | ||
| - | **[[4lui]] – MjOPD – ''Methanocaldococcus jannaschii''<br /> | ||
| - | **[[4dbd]] – SsOPD – ''Sulfolobus solftaricus'' <br /> | ||
| - | **[[4df0]] – TnOPD – ''Thermoproteus neutrophilus'' <br /> | ||
| - | **[[3ve9]] – MsOPD – ''Metallosphaera sedula'' <br /> | ||
| - | **[[3qw3]] – LdOPD – ''Leishmania donovani'' <br /> | ||
| - | **[[2za2]] - PfOPD - ''Plasmodium falciparum''<br /> | ||
| - | **[[1l2u]] - EcOPD - ''Escherichia coli''<br /> | ||
| - | **[[2cz5]], [[2czd]] - PhOPD - ''Pyrococcus horikoshii''<br /> | ||
| - | **[[3tfx]] - OPD - ''Lactobacillus acidophilus''<br /> | ||
| - | **[[3tr2]] - OPD - ''Coxiella burnetii''<br /> | ||
| - | **[[3r89]] - OPD - ''Anaerococcus prevorii''<br /> | ||
| - | **[[3v75]] - OPD - ''Streptomyces avermitilis''<br /> | ||
| - | **[[1vqt]] - OPD - ''Thermotoga maritima''<br /> | ||
| - | **[[3ldv]] - VcOPD - ''Vibrio cholerae''<br /> | ||
| - | **[[3ru6]] - OPD - ''Campylobacter jejuni''<br /> | ||
| - | **[[2yyt]], [[2yyu]] - OPD - ''Geobacillus kaustophilus''<br /> | ||
| - | **[[3g18]], [[3g1s]], [[3g1y]], [[3m41]], [[3m43]], [[3m44]], [[3m47]], [[3m5x]], [[3m5y]], [[3m5z]] – MtOPD (mutant) – ''Methanothermobacter thermautotrophicus''<br /> | ||
| - | |||
| - | *Uridine 5’-monophosphate synthase OPD subunit complex with reactants | ||
| - | |||
| - | **[[2v30]], [[2qcd]], [[2qcn]] - hOPD + UMP<br /> | ||
| - | **[[3ewy]] - hOPD (mutant) + UMP<br /> | ||
| - | **[[2qcg]], [[2qch]], [[3bgj]], [[3bk0]], [[3g3d]], [[3g3m]], [[3ewz]], [[3ex2]], [[3ex3]],[[3dbp]], [[3l0k]], [[3l0n]], [[3mo7]], [[3mw7]] - hOPD + UMP derivative<br /> | ||
| - | **[[2qcf]], [[2qcm]], [[2qcn]], [[3ewu]], [[3ewx]], [[3ex6]] - hOPD (mutant) + UMP derivative<br /> | ||
| - | **[[2qcl]] - hOPD (mutant) + OMP<br /> | ||
| - | **[[3ex1]] - hOPD + UMP derivative + UMP<br /> | ||
| - | **[[2qcl]] - yOPD (mutant) + OMP <br /> | ||
| - | **[[3gdl]] - yOPD + UMP derivative<br /> | ||
| - | **[[1dqx]], [[3gdt]] - yOPD (mutant) + UMP derivative<br /> | ||
| - | **[[4o8r]], [[4o11]], [[4nx5]], [[4nt0]], [[4nuw]], [[3wjw]], [[3wjx]], [[3wjy]], [[3wjz]], [[3w07]], [[3sgu]], [[3ssj]], [[3sw6]], [[3thq]], [[3wjw]], [[3wjx]], [[3w07]]] - MtOPD + UMP derivative<br /> | ||
| - | **[[1km1]], [[1km2]], [[1km3]], [[1km4]], [[1km5]], [[1kly]], [[1klz]], [[1km0]], [[1los]], [[2zz2]], [[2zz3]], [[2zz4]], [[2zz5]], [[2zz6]], [[2zz7]], [[3g1a]], [[3g1d]], [[3g1v]], [[3g1x]], [[3g22]], [[3g24]], [[3lld]], [[3llf]], [[3nq6]], [[3pbu]], [[3pbv]], [[3pbw]], [[3pby]], [[3pc0]], [[3sy5]], [[3wjy]], [[3wjz]], [[2e6y]] - MtOPD (mutant) + UMP derivative<br /> | ||
| - | **[[3wk0]], [[3wk1]], [[3wk2]], [[3wk3]] - MtOPD + OMP derivative<br /> | ||
| - | **[[1km6]], [[3g1f]], [[3g1h]] - MtOPD (mutant) + OMP derivative <br /> | ||
| - | **[[2za3]] - PfOPD + UMP <br /> | ||
| - | **[[1lp6]] - ArOPD + CMP - ''Archaea''<br /> | ||
| - | **[[1loq]] - ArOPD + UMP <br /> | ||
| - | **[[3uwq]] - VcOPD + UMP <br /> | ||
| - | **[[2cze]] - PhOPD + UMP <br /> | ||
| - | |||
| - | *Uridine 5’-monophosphate synthase OPD subunit complex with inhibitor | ||
| - | |||
| - | **[[3mi2]], [[4hib]], [[4hkp]] - hOPD + inhibitor<br /> | ||
| - | **[[3bvj]] - hOPD + XMP<br /> | ||
| - | **[[3bgg]], [[3ex4]] - hOPD + BMP<br /> | ||
| - | **[[4lw7]], [[3ltp]], [[4luj]], [[1lor]] – MtOPD + BMP <br /> | ||
| - | **[[4lc6]], [[4lc8]], [[4fx8]], [[4gc4]], [[4fx6]], [[4fxr]], [[3v1p]], [[1x1z]], [[3lht]], [[3lhu]], [[3lhv]], [[3lhw]], [[3lhy]], [[3lhz]], [[3li0]], [[3li1]], [[3lts]], [[3lty]], [[3lv5]], [[3lv6]], [[3m1z]], [[3nq7]], [[3nqa]], [[3nqc]], [[3nqd]], [[3nqe]], [[3nqf]], [[3nqg]], [[3nqm]], [[3p5y]], [[3p5z]], [[3p60]], [[3p61]], [[3qez]], [[3qf0]], [[3qmr]], [[3qms]], [[3qmt]], [[3rlu]], [[3rlv]], [[3siz]], [[3sj3]], [[3v1p]], [[4lw7]], [[2zz1]] – MtOPD (mutant) + BMP <br /> | ||
| - | **[[1lol]] - MtOPD + XMP <br /> | ||
| - | **[[3sec]] - MtOPD + inhibitor<br /> | ||
| - | **[[2czf]] - PhOPD + XMP<br /> | ||
| - | **[[4muz]] – AfOPD + BMP <br /> | ||
| - | **[[4luj]] – MjOPD + BMP <br /> | ||
| - | **[[4df1]] – TnOPD + BMP <br /> | ||
| - | **[[4dbe]] – SsOPD + BMP <br /> | ||
| - | **[[3ve7]] – MsOPD + BMP <br /> | ||
| - | **[[1jjk]] – EcOPD + BMP <br /> | ||
| - | **[[3vi2]] - PfOPD + inhibitor<br /> | ||
| - | **[[2za1]] - PfOPD + OMP <br /> | ||
| - | **[[1dqx]] – yOPD (mutant) + BMP <br /> | ||
| - | |||
| - | *Uridine 5’-monophosphate synthase OPRT subunit see orotate phosphoribosyltransferase in [[Phosphoribosyltransferase]] | ||
| - | |||
| - | }} | ||
==References== | ==References== | ||
<references /> | <references /> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Yablonski MJ, Pasek DA, Han BD, Jones ME, Traut TW. Intrinsic activity and stability of bifunctional human UMP synthase and its two separate catalytic domains, orotate phosphoribosyltransferase and orotidine-5'-phosphate decarboxylase. J Biol Chem. 1996 May 3;271(18):10704-8. PMID:8631878
- ↑ Grohmann K, Lauffer H, Lauenstein P, Hoffmann GF, Seidlitz G. Hereditary orotic aciduria with epilepsy and without megaloblastic anemia. Neuropediatrics. 2015 Apr;46(2):123-5. doi: 10.1055/s-0035-1547341. Epub 2015 Mar, 10. PMID:25757096 doi:http://dx.doi.org/10.1055/s-0035-1547341
- ↑ Schwenger B, Schober S, Simon D. DUMPS cattle carry a point mutation in the uridine monophosphate synthase gene. Genomics. 1993 Apr;16(1):241-4. PMID:8486364 doi:http://dx.doi.org/10.1006/geno.1993.1165
- ↑ Wittmann JG, Heinrich D, Gasow K, Frey A, Diederichsen U, Rudolph MG. Structures of the human orotidine-5'-monophosphate decarboxylase support a covalent mechanism and provide a framework for drug design. Structure. 2008 Jan;16(1):82-92. PMID:18184586 doi:http://dx.doi.org/10.1016/j.str.2007.10.020