Sandbox Reserved 1480
From Proteopedia
(New page: {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> ==Your Heading Here (maybe something like 'Structure')== <StructureSection load='1stp' size='340' side='right' caption=...) |
|||
| (67 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == | + | ==Structure of the protein LHPP== |
| - | <StructureSection load=' | + | <StructureSection load='2x4d' size='340' side='right' caption='LHPP structure' scene=''> |
| - | + | ||
| - | + | ||
== Function == | == Function == | ||
| + | <div align='justified'>The <scene name='80/802654/2x4d/1'>human protein LHPP</scene> or phospholysine phosphohistidine inorganic pyrophosphate phosphatase (hLHPP) is a phosphatase with an in vitro activity towards inorganic pyrophosphate, imidodiphosphate, 3‑phosphohistidine and 6‑phospholysine. hLHPP is part of the haloacid dehalogenase family (HAD) . It is a protein of 271 amino acids which is encoded by a seven exon gene positioned on the chromosome 10. The enzyme acts more effectively on N-P bonds than O-P bonds, and is found on the nucleus and in the cytoplasm.</div> | ||
| + | |||
| + | [[Image:HAD.PNG]] | ||
| + | |||
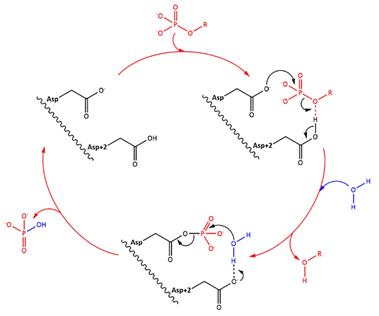
| + | ''Fig 1. Simplified catalytic mechanism of HAD phosphatases. Asp acts as a nucleophile and Asp+2 as a acid/base. The phosphorylated substrate and the respective leaving groups are shown in red, and the water nucleophile is in blue. (Source: Do metabolic HAD phosphatases moonlight as protein phosphatases?, A. Gohla, 2018). In the human LHPP, the Asp+2 is replaced by a Serine.'' | ||
| + | |||
| + | LHPP is expressed in brain (highest expression level in C1 segment of cervical spinal cord), and at lower levels in liver and kidney. | ||
| + | Physiological LHPP substrates have not yet been reported. | ||
| + | Although the subcellular localization of LHPP is unknown, the protein seems to interact with ATP synthase subunits, and may be involved in mitochondrial oxidative phosphorylation. | ||
== Disease == | == Disease == | ||
| + | |||
| + | Different studies have shown that LHPP is a risk factor in major depressive disorder. Indeed, single nucleotide polymorphisms (SNPs) are linked with serotonin receptor 1A-1019C > G genotyp in two different populations. Enhanced expression of the HTR1A 1A-1019C > G autoreceptor is implicated in the reduction of serotonergic neurotransmission, which is considered to be one of the primary defects in depression, resulting in stress and vulnerability. | ||
| + | |||
| + | It has also been shown that LHPP is a histidine phosphatase and a tumour suppressor in hepatocellular carcinoma that removes histidine-linked phosphate groups from proteins. The absence of LHPP promotes the growth of tumors via increasing histidine-phosphorylated proteins. This study shown that LHPP was downregulated in hepatocellular carcinoma, but also that two histidine kinases (NME1 and NME2) were upregulated. Thus, the regulation of protein phosphorylation is important for the integrity of the cell and when deregulated could lead to tumorigenesis. Collectively, this study provides unprecedented hints on cellular functions of LHPP, and shows that LHPP inactivation together with high NME1/2 expression and - activity is a key tumorigenic event in hepatocellular carcinomas. | ||
| + | |||
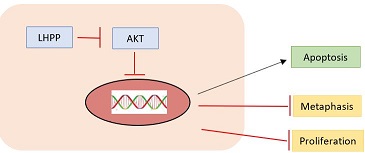
| + | LHPP regulates AKT to control the growth of liver cancer because AKT can block apoptosis and thereby promote cell survival and is considered as an essential factor in the development of many types of cancer. | ||
| + | Moreover, LHPP apparently inhibited the metastasis of cervical cancer cells, as evidenced by the reduced expressions of MMP-2, MMP-9, TGF-β1, Fibronectin, N-cadherin and Vimentin, and the enhanced E-cadherin. | ||
| + | |||
| + | [[Image:LHPP.jpg]] | ||
| + | ''Fig 2. Action mode of LHPP on the kinase AKT'' | ||
| + | |||
| + | The researchers led by Prof. Michael N. Hall from the Biozentrum, University of Basel, report in “Nature” that LHPP can also serve as a biomarker for the diagnosis and prognosis of liver cancer. | ||
== Relevance == | == Relevance == | ||
| + | Liver cancer is the second leading cause of cancer causing the most deaths. Hepatocellular carcinoma (HCC) represents approximately 90% of primary liver cancer cases. In order to finally find a solution to this disease that is ravaging thousands of people, the discovery of the role of the LHPP protein as a suppressor tumor is essential to hopefully cure liver cancer patients by turning off the uncontrolled growth of cells. | ||
| + | |||
| + | Moreover, the mode of action of the protein could also be relevant for other cancers types. | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | [[Image:Capture.jpg]] | |
| + | |||
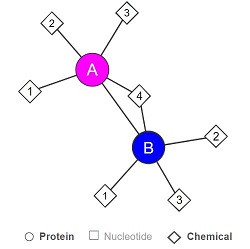
| + | ''Fig 3. Simplified structure of LHPP. 1: Pyrophosphate, 2: Magnesium ion, 3: 4-(2-hydroxyethyl)-1-Piperazine ethanesulfonic acid, 4: Glycerol. A, B: the two protein subunits of the homodimer. Source : Uniprot, 2018'' | ||
| + | |||
| + | |||
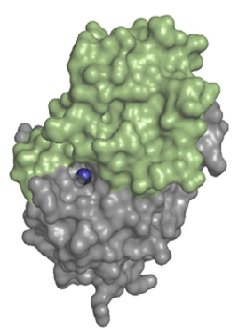
| + | LHPP forms a homodimer in solution. Each <scene name='80/802654/2x4d_monomer/10'>monomer</scene> of hLHPP has two domains, one catalytic domain with a <scene name='80/802654/Mg_ion/4'>phosphoric acid and a magnesium ion</scene> (Mg2+) on the <scene name='80/802654/Catalityc_site/5'>active site</scene> and a large C2a-type cap domain which control the accessibility to the active site (see Fig 4). Despite the large cap, the enzyme is not only able to act on small metabolites, but also on phosphoproteins. | ||
| + | |||
| + | The <scene name='80/802654/Catalityc_site_aa/7'>amino acids</scene> involved in the catalytic center are number 18 to 22, from 55 to 60, 155, 190, from 214 to 216, 219 and 220 corresponding respectively to DISGV, TNESAA, G, K, GDD, G and D. | ||
| + | |||
| + | [[Image:2 domains.png]] | ||
| + | |||
| + | ''Fig 4. Structure of a monomer of hLHPP. The catalytic domains is shown in gray, and the C2a-type cap domains is colored in green. The essential Mg2+ cofactor in the active site is shown as a blue sphere. Source : Do metabolic HAD phosphatases moonlight as protein phosphatases? Antje Gohla. (2018) [BBA - Molecular Cell Research]'' | ||
| + | |||
| + | |||
| + | Full crystallographic information is available from [https://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2x4d OCA] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| - | + | ||
| + | "Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase." Yokoi F., Hiraishi H., Izuhara K. | ||
| + | J. Biochem. 133:607-614(2003) [PubMed] [Europe PMC] [Abstract] | ||
| + | |||
| + | "The protein histidine phosphatase LHPP is a tumour suppressor." Sravanth K. Hindupur, Marco Colombi, Stephen R. Fuhs, Matthias S. Matter, Yakir Guri, Kevin Adam, Marion Cornu, Salvatore Piscuoglio, Charlotte K. Y. Ng, Charles Betz, Dritan Liko, Luca Quagliata, Suzette Moes, Paul Jenoe, Luigi M. Terracciano, Markus H. Heim, Tony Hunter & Michael N. Hall. (2018) [PubMed] [Main] | ||
| + | |||
| + | "Do metabolic HAD phosphatases moonlight as protein phosphatases?." Antje Gohla. (2018) [BBA - Molecular Cell Research] | ||
| + | |||
| + | "Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT", (2018), [PubMed] [Main] | ||
| + | |||
| + | "The human LHPP page on Uniprot.org [https://www.uniprot.org/uniprot/Q9H008]" | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Structure of the protein LHPP
| |||||||||||
References
"Molecular cloning of a cDNA for the human phospholysine phosphohistidine inorganic pyrophosphate phosphatase." Yokoi F., Hiraishi H., Izuhara K. J. Biochem. 133:607-614(2003) [PubMed] [Europe PMC] [Abstract]
"The protein histidine phosphatase LHPP is a tumour suppressor." Sravanth K. Hindupur, Marco Colombi, Stephen R. Fuhs, Matthias S. Matter, Yakir Guri, Kevin Adam, Marion Cornu, Salvatore Piscuoglio, Charlotte K. Y. Ng, Charles Betz, Dritan Liko, Luca Quagliata, Suzette Moes, Paul Jenoe, Luigi M. Terracciano, Markus H. Heim, Tony Hunter & Michael N. Hall. (2018) [PubMed] [Main]
"Do metabolic HAD phosphatases moonlight as protein phosphatases?." Antje Gohla. (2018) [BBA - Molecular Cell Research]
"Down-regulation of LHPP in cervical cancer influences cell proliferation, metastasis and apoptosis by modulating AKT", (2018), [PubMed] [Main]
"The human LHPP page on Uniprot.org [1]"