|
|
| (2 intermediate revisions not shown.) |
| Line 1: |
Line 1: |
| | | | |
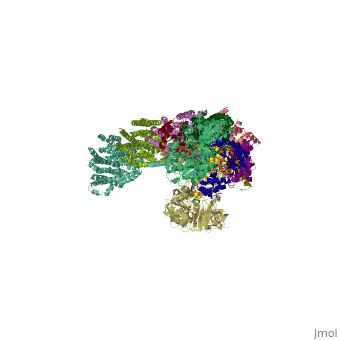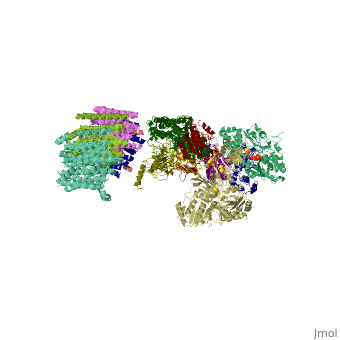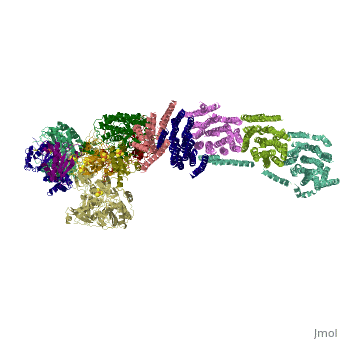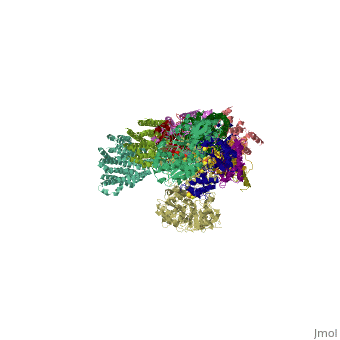
| | ==Crystal structure of respiratory complex I from Thermus thermophilus== | | ==Crystal structure of respiratory complex I from Thermus thermophilus== |
| - | <StructureSection load='3m9s' size='340' side='right' caption='[[3m9s]], [[Resolution|resolution]] 4.50Å' scene=''> | + | <StructureSection load='3m9s' size='340' side='right'caption='[[3m9s]], [[Resolution|resolution]] 4.50Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[3m9s]] is a 26 chain structure with sequence from [http://en.wikipedia.org/wiki/Thermus_thermophilus Thermus thermophilus]. The December 2011 RCSB PDB [http://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Complex I'' by David Goodsell is [http://dx.doi.org/10.2210/rcsb_pdb/mom_2011_12 10.2210/rcsb_pdb/mom_2011_12]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3M9S OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3M9S FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[3m9s]] is a 20 chain structure with sequence from [https://en.wikipedia.org/wiki/Thermus_thermophilus_HB8 Thermus thermophilus HB8]. The December 2011 RCSB PDB [https://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''Complex I'' by David Goodsell is [https://dx.doi.org/10.2210/rcsb_pdb/mom_2011_12 10.2210/rcsb_pdb/mom_2011_12]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3M9S OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3M9S FirstGlance]. <br> |
| - | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=FES:FE2/S2+(INORGANIC)+CLUSTER'>FES</scene>, <scene name='pdbligand=FMN:FLAVIN+MONONUCLEOTIDE'>FMN</scene>, <scene name='pdbligand=SF4:IRON/SULFUR+CLUSTER'>SF4</scene></td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 4.5Å</td></tr> |
| - | <tr id='NonStdRes'><td class="sblockLbl"><b>[[Non-Standard_Residue|NonStd Res:]]</b></td><td class="sblockDat"><scene name='pdbligand=UNK:UNKNOWN'>UNK</scene></td></tr>
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=FES:FE2/S2+(INORGANIC)+CLUSTER'>FES</scene>, <scene name='pdbligand=FMN:FLAVIN+MONONUCLEOTIDE'>FMN</scene></td></tr> |
| - | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[3m9c|3m9c]]</td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3m9s FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3m9s OCA], [https://pdbe.org/3m9s PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3m9s RCSB], [https://www.ebi.ac.uk/pdbsum/3m9s PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3m9s ProSAT]</span></td></tr> |
| - | <tr id='activity'><td class="sblockLbl"><b>Activity:</b></td><td class="sblockDat"><span class='plainlinks'>[http://en.wikipedia.org/wiki/NADH_dehydrogenase_(quinone) NADH dehydrogenase (quinone)], with EC number [http://www.brenda-enzymes.info/php/result_flat.php4?ecno=1.6.5.11 1.6.5.11] </span></td></tr>
| + | |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=3m9s FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3m9s OCA], [http://pdbe.org/3m9s PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=3m9s RCSB], [http://www.ebi.ac.uk/pdbsum/3m9s PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=3m9s ProSAT]</span></td></tr> | + | |
| | </table> | | </table> |
| | == Function == | | == Function == |
| - | [[http://www.uniprot.org/uniprot/NQO6_THET8 NQO6_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP.[HAMAP-Rule:MF_01356] [[http://www.uniprot.org/uniprot/NQO3_THET8 NQO3_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. [[http://www.uniprot.org/uniprot/NQO9_THET8 NQO9_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The role of the nqo9 subunit appears to provide a 'connecting chain' of two clusters between cluster N5 and the terminal cluster N2, and to stabilize the structure of the complex by interacting with other subunits.[HAMAP-Rule:MF_01351] [[http://www.uniprot.org/uniprot/NQO2_THET8 NQO2_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. [[http://www.uniprot.org/uniprot/NQO15_THET8 NQO15_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The nqo15 subunit has probably a role in complex stabilization, and may be also involved in the storage of iron for iron-sulfur cluster regeneration in the complex.<ref>PMID:16469879</ref> [[http://www.uniprot.org/uniprot/NQO1_THET8 NQO1_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The nqo1 subunit contains the NADH-binding site and the primary electron acceptor FMN. [[http://www.uniprot.org/uniprot/NQO4_THET8 NQO4_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The nqo4 subunit may contain the quinone-binding site.[HAMAP-Rule:MF_01358] [[http://www.uniprot.org/uniprot/NQO5_THET8 NQO5_THET8]] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The nqo5 subunit may be involved in the stabilization of the complex.[HAMAP-Rule:MF_01357] | + | [https://www.uniprot.org/uniprot/NQO1_THET8 NQO1_THET8] NDH-1 shuttles electrons from NADH, via FMN and iron-sulfur (Fe-S) centers, to quinones in the respiratory chain. The immediate electron acceptor for the enzyme in this species is menaquinone. Couples the redox reaction to proton translocation (for every two electrons transferred, four hydrogen ions are translocated across the cytoplasmic membrane), and thus conserves the redox energy in a proton gradient required for the synthesis of ATP. The nqo1 subunit contains the NADH-binding site and the primary electron acceptor FMN. |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 17: |
Line 15: |
| | <jmolCheckbox> | | <jmolCheckbox> |
| | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/m9/3m9s_consurf.spt"</scriptWhenChecked> | | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/m9/3m9s_consurf.spt"</scriptWhenChecked> |
| - | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview01.spt</scriptWhenUnchecked> | + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> |
| | <text>to colour the structure by Evolutionary Conservation</text> | | <text>to colour the structure by Evolutionary Conservation</text> |
| | </jmolCheckbox> | | </jmolCheckbox> |
| Line 34: |
Line 32: |
| | ==See Also== | | ==See Also== |
| | *[[NADH-quinone oxidoreductase|NADH-quinone oxidoreductase]] | | *[[NADH-quinone oxidoreductase|NADH-quinone oxidoreductase]] |
| - | *[[NADH:ubiquinone oxidoreductase|NADH:ubiquinone oxidoreductase]] | |
| | == References == | | == References == |
| | <references/> | | <references/> |
| Line 40: |
Line 37: |
| | </StructureSection> | | </StructureSection> |
| | [[Category: Complex I]] | | [[Category: Complex I]] |
| | + | [[Category: Large Structures]] |
| | [[Category: RCSB PDB Molecule of the Month]] | | [[Category: RCSB PDB Molecule of the Month]] |
| - | [[Category: Thermus thermophilus]] | + | [[Category: Thermus thermophilus HB8]] |
| - | [[Category: Baradaran, R]] | + | [[Category: Baradaran R]] |
| - | [[Category: Efremov, R G]] | + | [[Category: Efremov RG]] |
| - | [[Category: Sazanov, L A]] | + | [[Category: Sazanov LA]] |
| - | [[Category: Complex i]]
| + | |
| - | [[Category: Electron transport]]
| + | |
| - | [[Category: Membrane protein]]
| + | |
| - | [[Category: Oxidoreductase]]
| + | |
| - | [[Category: Respiratory chain]]
| + | |