User:Estelle Blochouse/ Sandbox 1497
From Proteopedia
< User:Estelle Blochouse(Difference between revisions)
| (132 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | == | + | ==Multicopper Oxidase CueO (4e9s)== |
| - | + | ||
| - | + | <StructureSection load='4e9s' size='340' side='right' caption='[[4e9s]], [[Resolution|resolution]] 1.06Å' scene=''> | |
| - | + | ||
| - | Copper is an important metal involed in multiple enzyme catalysed reactions as a cofactor. | ||
| + | Copper is one of the most important metallic cofactor involved in enzyme catalysed reactions. In living organisms, its function is related to its redox properties. However, copper is toxic at all concentration, therefore it needs to be strictly controlled by molecular mechanisms.<ref>EMBL-EBI, Family: Cu-oxidase (PF00394), Summary: Multicopper oxidase, http://pfam.xfam.org/family/Cu-oxidase</ref> | ||
== Function == | == Function == | ||
| - | Multicopper oxidases are involved in copper homeostasis. | + | Multicopper oxidases are enzymes involved in copper homeostasis. Indeed, free copper present in a cell (so, not bounded to a protein) is harmful and can cause cellular damage. It needs to be regulated, it is why multicopper oxydases CueO 4e9s is essential. |
| + | |||
| + | Multicopper oxidase acts probably for the detoxification of copper present in the periplasmic space. It oxidizes the Cu+ into Cu2+ and prevents its uptake by the cytoplasm. It also possesses a phenoloxidase and a ferroxidase activity which can be involved in the prevention of oxidative damage.<ref><span class='plainlinks'>[https://www.uniprot.org/uniprot/P36649 UniProtKB]</span></ref> | ||
| + | |||
Multicopper oxidase might also be involved in the regulation of metal transport. | Multicopper oxidase might also be involved in the regulation of metal transport. | ||
| + | |||
| + | Multicopper oxidases are able to oxidise their substrates thanks to their particular structure. They own four copper ions (Cu1001, Cu1002, Cu1003 and C1004) shared between two important areas : the mononuclear copper center (Cu1001) and the trinuclear copper center (Cu1002, Cu1003 and Cu1004). | ||
| + | |||
| + | |||
| + | == Mechanism == | ||
| + | Multicopper oxidase catalyzes the oxidation of different substrates by reducing O2 into H2O without releasing activated oxygen species (H2O2). | ||
| + | |||
| + | Three different copper centers exist which can be differentiated by spectroscopy: Type 1 or blue (<scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene>), type 2 or normal and type 3 or coupled binuclear.<ref>Messerschmidt A, Huber R, The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships, Eur. J. Biochem. 187, January 1990</ref><ref>Ouzounis C, Sander C, A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins, FEBS Lett. volume 279, February 1991</ref>. | ||
| + | Multicopper oxidase contains these 3 types of copper ions. They are all involved in the transfer of electrons from the substrate to the dioxygen. The first type of copper, type 1 (Cu1001) mediates the electron transfer from the substrate to the other coppers. The <scene name='pdbligand=CU:COPPER+(II)+ION'>mononoclear copper center</scene> formed by the three other coppers is the key element for the oxygen reduction. It contains a type 2 copper (Cu1004) and two type 3 copper ions, binuclear ions (Cu1002 and Cu1003). The final electron acceptor, O2, is bound to this last type of copper and is reduced into two molecules of water thanks to the transfer of four electrons allowed by the oxidation of the four copper ions.<ref>Hirofumi Komori, Ryosuke | ||
| + | Sugiyama, Kunishige Kataoka, | ||
| + | Kentaro Miyazaki, Yoshiki | ||
| + | Higuchib, and Takeshi Sakurai, New insights into the catalytic active-site structure | ||
| + | of multicopper oxidases, Biological | ||
| + | Crystallography, 6 December 2013 doi:10.1107/S1399004713033051</ref> | ||
== Disease == | == Disease == | ||
| - | If the amino acids 500 and 501 are mutated from CH to SR, | + | If the amino acids 500 and 501 are mutated from CH to SR, there is a loss of resistance to copper which is highly harmful for the bacteria. |
| + | Oxygen deprivation also leads to protein degradation. | ||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>Multicopper Oxidase CueO [[4e9s]] is a protein containing one chain with sequence from [http://en.wikipedia.org/wiki/Ecoli Ecoli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=4E9S OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4E9S FirstGlance]. <br> | ||
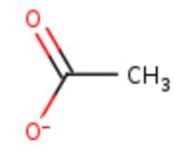
| + | </td></tr><tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat"><scene name='pdbligand=ACT:ACETATE+ION'>Acetate ion</scene>[[Image:Act.jpg|thumb|middle|'''Figure 1:''' Acetate ion.<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]], <scene name='pdbligand=CU:COPPER+(II)+ION'>Copper</scene></td></tr> | ||
| + | <tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[4e9p|4e9p]], [[4e9q|4e9q]], [[4e9r|4e9r]], [[4e9t|4e9t]]</td></tr> | ||
| + | <tr id='gene'><td class="sblockLbl"><b>[[Gene|Gene:]]</b></td><td class="sblockDat">cueO, yacK, b0123, JW0119 ([http://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?mode=Info&srchmode=5&id=83333 ECOLI])</td></tr> | ||
| + | <tr id='function'><td class="sblockLbl"><b>Function:</b></td><td class="sblockDat">binding of a metal ion</td></tr> | ||
| + | <tr id='process'><td class="sblockLbl"><b>Process:</b></td><td class="sblockDat">oxidation-reduction</td></tr> | ||
| + | <tr id='position'><td class="sblockLbl"><b>Position:</b></td><td class="sblockDat">bound at the outer membrane in the periplasmic space</td></tr> | ||
| + | <tr id='chain'><td class="sblockLbl"><b>Chain:</b></td><td class="sblockDat">A</td></tr> | ||
| + | <tr id='sequence domain'><td class="sblockLbl"><b>Sequence domain:</b></td><td class="sblockDat">Cupredoxin, Multicopper oxidase type 1, type 2, type 3 and a copper-binding site</td></tr> | ||
| + | <tr id='number of amino acids'><td class="sblockLbl"><b>Number of amino acids:</b></td><td class="sblockDat">489</td></tr> | ||
| + | <tr id='production and extraction'><td class="sblockLbl"><b>Production and extraction:</b></td><td class="sblockDat">Escherichia Coli</td></tr> | ||
| + | <tr id='initial gene'><td class="sblockLbl"><b>Initial gene:</b></td><td class="sblockDat">sequence from amino acid 29 to amino acid 516 from P36649</td></tr> | ||
| + | <tr id='specific region'><td class="sblockLbl"><b>Specific region:</b></td><td class="sblockDat">contain a methionine rich region (aa355 to aa400) that can be important for copper tolerance in bacteria</td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=4e9s FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=4e9s OCA], [http://pdbe.org/4e9s PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=4e9s RCSB], [http://www.ebi.ac.uk/pdbsum/4e9s PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=4e9s ProSAT], [https://www.ebi.ac.uk/pdbe/entry/pdb/4E9S EMBL-EBI]</span></td></tr> | ||
| + | </table> | ||
| + | |||
| + | |||
| + | <table><tr><td colspan='2'>In the chain, five ligands are present: an acetate ion (C2H3O2) and four copper ions. | ||
| + | Even if the trinuclear copper center is deeply buried inside the protein, the channel is dynamic enough to allow the acetate ion to approach. It has been noticed that adding acetate ion is enhancing the enzymatic activity of the protein. <br> | ||
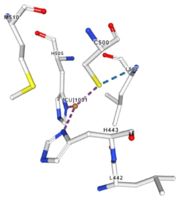
| + | </td></tr><tr><td>[[Image:Cu1001.jpg|thumb|left|'''Figure 2:''' Copper 1001<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]]</td><td>Cu1001: The copper ion is bound with H443, H505 and C500 thanks to three metallic interactions. The structure is stabilised by hydrogen bounds with L502, M510.</td></tr> | ||
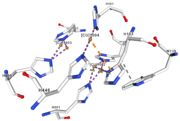
| + | <tr><td>[[Image:Cu1002.jpg|thumb|left|'''Figure 3:''' Copper 1002<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]]</td><td>Cu1002: The binuclear copper ion is bound with three histidines : H501, H103, and H141. Each bound is a double metallic interaction because the two nucleus of the binuclear ion are bound. The structure is stabilised by hydrophobic contact between H141 and W139</td></tr> | ||
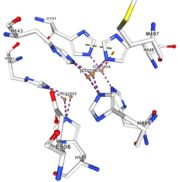
| + | <tr><td>[[Image:Cu1003.jpg|thumb|left|'''Figure 4:''' Copper 1003<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]]</td><td>Cu1003: The binuclear copper ion is bound with three with three histidines : H499, H143, and H448. Each bound is a double metallic interaction. The structure is stabilised by Pi interactions with Cu1004 and H101</td></tr> | ||
| + | <tr><td>[[Image:Cu1004.jpg|thumb|left|'''Figure 5:''' Copper 1004<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]]</td><td>Cu1004: The copper ion is bound by metallic interaction with two histidines : H101 and H446, and with Act1005. The structure is stabilised by Pi interactions between H446 and two other histidines : H103 and H448. Cu1004 bounds also by Pi interactions with H103 and H448. Moreover, their are Pi interactions between H113 and H446</td></tr> | ||
| + | <tr><td>[[Image:Act10051.jpg|thumb|left|'''Figure 6:''' Acetate ion 1005<ref><span class='plainlinks'>[https://www.rcsb.org/structure/4e9s RCBS PDB]</span></ref>]]</td><td>Act1005: The acetate ion is linked by hydrogen bonds to G104 and G449.</td></tr> | ||
| + | </table> | ||
| + | |||
| + | Cu1002, Cu1003, Cu1004 and Act1005 are very closed in the tridimensionnal structure of the protein. The three copper ions form the trinuclear copper center. The bound lengths are nearly equal so the three copper are arranged as a triangle. | ||
| + | |||
| + | |||
| + | <table><tr><td colspan='2'>The amino acid sequence is: <br> | ||
| + | </td></tr> | ||
| + | <tr><td>29</td><td>30</td><td>31</td><td>32</td><td>33</td><td>34</td><td>35</td><td>36</td><td>37</td><td>38</td><td>39</td><td>40</td><td>41</td><td>42</td><td>43</td><td>44</td><td>45</td><td>46</td><td>47</td><td>48</td><td>49</td><td>50</td><td>51</td><td>52</td><td>53</td><td>54</td><td>55</td><td>56</td><td>57</td><td>58</td></tr> | ||
| + | <tr><td>A</td><td>E</td><td>R</td><td>P</td><td>T</td><td>L</td><td>P</td><td>I</td><td>P</td><td>D</td><td>L</td><td>L</td><td>T</td><td>T</td><td>D</td><td>A</td><td>R</td><td>N</td><td>R</td><td>I</td><td>Q</td><td>L</td><td>T</td><td>I</td><td>G</td><td>A</td><td>G</td><td>Q</td><td>S</td><td>T</td></tr> | ||
| + | <tr><td>59</td><td>60</td><td>61</td><td>62</td><td>63</td><td>64</td><td>65</td><td>66</td><td>67</td><td>68</td><td>69</td><td>70</td><td>71</td><td>72</td><td>73</td><td>74</td><td>75</td><td>76</td><td>77</td><td>78</td><td>79</td><td>80</td><td>81</td><td>82</td><td>83</td><td>84</td><td>85</td><td>86</td><td>87</td><td>88</td></tr> | ||
| + | <tr><td>F</td><td>G</td><td>G</td><td>K</td><td>T</td><td>A</td><td>T</td><td>T</td><td>W</td><td>G</td><td>Y</td><td>N</td><td>G</td><td>N</td><td>L</td><td>L</td><td>G</td><td>P</td><td>A</td><td>V</td><td>K</td><td>L</td><td>Q</td><td>R</td><td>G</td><td>K</td><td>A</td><td>V</td><td>T</td><td>V</td></tr> | ||
| + | <tr><td>89</td><td>90</td><td>91</td><td>92</td><td>93</td><td>94</td><td>95</td><td>96</td><td>97</td><td>98</td><td>99</td><td>100</td><td bgcolor="#999999">101</td><td>102</td><td class="sblockLbl">103</td><td bgcolor="#CCFFFF">104</td><td>105</td><td>106</td><td>107</td><td>108</td><td>109</td><td>110</td><td>111</td><td>112</td><td>113</td><td>114</td><td>115</td><td>116</td><td>117</td><td>118</td></tr> | ||
| + | <tr><td>D</td><td>I</td><td>Y</td><td>N</td><td>Q</td><td>L</td><td>T</td><td>E</td><td>E</td><td>T</td><td>T</td><td>L</td><td bgcolor="#999999">H</td><td>W</td><td class="sblockLbl">H</td><td bgcolor="#CCFFFF">G</td><td>L</td><td>E</td><td>V</td><td>P</td><td>G</td><td>E</td><td>V</td><td>D</td><td>G</td><td>G</td><td>P</td><td>Q</td><td>G</td><td>I</td></tr> | ||
| + | <tr><td>119</td><td>120</td><td>121</td><td>122</td><td>123</td><td>124</td><td>125</td><td>126</td><td>127</td><td>128</td><td>129</td><td>130</td><td>131</td><td>132</td><td>133</td><td>134</td><td>135</td><td>136</td><td>137</td><td>138</td><td>139</td><td>140</td><td class="sblockLbl">141</td><td>142</td><td bgcolor="#FF0000">143</td><td>144</td><td>145</td><td>146</td><td>147</td><td>148</td></tr> | ||
| + | <tr><td>I</td><td>P</td><td>P</td><td>G</td><td>G</td><td>K</td><td>R</td><td>S</td><td>V</td><td>T</td><td>L</td><td>N</td><td>V</td><td>D</td><td>Q</td><td>P</td><td>A</td><td>A</td><td>T</td><td>C</td><td>W</td><td>F</td><td class="sblockLbl">H</td><td>P</td><td bgcolor="#FF0000">H</td><td>Q</td><td>H</td><td>G</td><td>K</td><td>T</td></tr> | ||
| + | <tr><td>149</td><td>150</td><td>151</td><td>152</td><td>153</td><td>154</td><td>155</td><td>156</td><td>157</td><td>158</td><td>159</td><td>160</td><td>161</td><td>162</td><td>163</td><td>164</td><td>165</td><td>166</td><td>167</td><td>168</td><td>169</td><td>170</td><td>171</td><td>172</td><td>173</td><td>174</td><td>175</td><td>176</td><td>177</td><td>178</td></tr> | ||
| + | <tr><td>G</td><td>R</td><td>Q</td><td>V</td><td>A</td><td>M</td><td>G</td><td>L</td><td>A</td><td>G</td><td>L</td><td>V</td><td>V</td><td>I</td><td>E</td><td>D</td><td>D</td><td>E</td><td>I</td><td>L</td><td>K</td><td>L</td><td>M</td><td>L</td><td>P</td><td>K</td><td>Q</td><td>W</td><td>G</td><td>I</td></tr> | ||
| + | <tr><td>179</td><td>180</td><td>181</td><td>182</td><td>183</td><td>184</td><td>185</td><td>186</td><td>187</td><td>188</td><td>189</td><td>190</td><td>191</td><td>192</td><td>193</td><td>194</td><td>195</td><td>196</td><td>197</td><td>198</td><td>199</td><td>200</td><td>201</td><td>202</td><td>203</td><td>204</td><td>205</td><td>206</td><td>207</td><td>208</td></tr> | ||
| + | <tr><td>D</td><td>D</td><td>V</td><td>P</td><td>V</td><td>I</td><td>V</td><td>Q</td><td>D</td><td>K</td><td>K</td><td>F</td><td>S</td><td>A</td><td>D</td><td>G</td><td>Q</td><td>I</td><td>D</td><td>Y</td><td>Q</td><td>L</td><td>D</td><td>V</td><td>M</td><td>T</td><td>A</td><td>A</td><td>V</td><td>G</td></tr> | ||
| + | <tr><td>209</td><td>210</td><td>211</td><td>212</td><td>213</td><td>214</td><td>215</td><td>216</td><td>217</td><td>218</td><td>219</td><td>220</td><td>221</td><td>222</td><td>223</td><td>224</td><td>225</td><td>226</td><td>227</td><td>228</td><td>229</td><td>230</td><td>231</td><td>232</td><td>233</td><td>234</td><td>235</td><td>236</td><td>237</td><td>238</td></tr> | ||
| + | <tr><td>W</td><td>F</td><td>G</td><td>D</td><td>T</td><td>L</td><td>L</td><td>T</td><td>N</td><td>G</td><td>A</td><td>I</td><td>Y</td><td>P</td><td>Q</td><td>H</td><td>A</td><td>A</td><td>P</td><td>R</td><td>G</td><td>W</td><td>L</td><td>R</td><td>L</td><td>R</td><td>L</td><td>L</td><td>N</td><td>G</td></tr> | ||
| + | <tr><td>239</td><td>240</td><td>241</td><td>242</td><td>243</td><td>244</td><td>245</td><td>246</td><td>247</td><td>248</td><td>249</td><td>250</td><td>251</td><td>252</td><td>253</td><td>254</td><td>255</td><td>256</td><td>257</td><td>258</td><td>259</td><td>260</td><td>261</td><td>262</td><td>263</td><td>264</td><td>265</td><td>266</td><td>267</td><td>268</td><tr/> | ||
| + | <tr><td>C</td><td>N</td><td>A</td><td>R</td><td>S</td><td>L</td><td>N</td><td>F</td><td>A</td><td>T</td><td>S</td><td>D</td><td>N</td><td>R</td><td>P</td><td>L</td><td>Y</td><td>V</td><td>I</td><td>A</td><td>S</td><td>D</td><td>G</td><td>G</td><td>L</td><td>L</td><td>P</td><td>E</td><td>P</td><td>V</td></tr> | ||
| + | <tr><td>269</td><td>270</td><td>271</td><td>272</td><td>273</td><td>274</td><td>275</td><td>276</td><td>277</td><td>278</td><td>279</td><td>280</td><td>281</td><td>282</td><td>283</td><td>284</td><td>285</td><td>286</td><td>287</td><td>288</td><td>289</td><td>290</td><td>291</td><td>292</td><td>293</td><td>294</td><td>295</td><td>296</td><td>297</td><td>298</td></tr> | ||
| + | <tr><td>K</td><td>V</td><td>S</td><td>E</td><td>L</td><td>P</td><td>V</td><td>L</td><td>M</td><td>G</td><td>E</td><td>R</td><td>F</td><td>E</td><td>V</td><td>L</td><td>V</td><td>E</td><td>V</td><td>N</td><td>D</td><td>N</td><td>K</td><td>P</td><td>F</td><td>D</td><td>L</td><td>V</td><td>T</td><td>L</td></tr> | ||
| + | <tr><td>299</td><td>300</td><td>301</td><td>302</td><td>303</td><td>304</td><td>305</td><td>306</td><td>307</td><td>308</td><td>309</td><td>310</td><td>311</td><td>312</td><td>313</td><td>314</td><td>315</td><td>316</td><td>317</td><td>318</td><td>319</td><td>310</td><td>321</td><td>322</td><td>323</td><td>324</td><td>325</td><td>326</td><td>327</td><td>328</td></tr> | ||
| + | <tr><td>P</td><td>V</td><td>S</td><td>Q</td><td>M</td><td>G</td><td>M</td><td>A</td><td>I</td><td>A</td><td>P</td><td>F</td><td>D</td><td>K</td><td>P</td><td>H</td><td>P</td><td>V</td><td>M</td><td>R</td><td>I</td><td>Q</td><td>P</td><td>I</td><td>A</td><td>I</td><td>S</td><td>A</td><td>S</td><td>G</td></tr> | ||
| + | <tr><td>329</td><td>330</td><td>331</td><td>332</td><td>333</td><td>334</td><td>335</td><td>336</td><td>337</td><td>338</td><td>339</td><td>340</td><td>341</td><td>342</td><td>343</td><td>344</td><td>345</td><td>346</td><td>347</td><td>348</td><td>349</td><td>350</td><td>351</td><td>352</td><td>353</td><td>354</td><td>355</td><td>356</td><td>357</td><td>358</td></tr> | ||
| + | <tr><td>A</td><td>L</td><td>P</td><td>D</td><td>T</td><td>L</td><td>S</td><td>S</td><td>L</td><td>P</td><td>A</td><td>L</td><td>P</td><td>S</td><td>L</td><td>E</td><td>G</td><td>L</td><td>T</td><td>V</td><td>R</td><td>K</td><td>L</td><td>Q</td><td>L</td><td>S</td><td>M</td><td>D</td><td>P</td><td>M</td></tr> | ||
| + | <tr<<td>359</td><td>360</td><td>361</td><td>362</td><td>363</td><td>364</td><td>365</td><td>366</td><td>367</td><td>368</td><td>369</td><td>370</td><td>371</td><td>372</td><td>373</td><td>374</td><td>375</td><td>376</td><td>377</td><td>378</td><td>379</td><td>380</td><td>381</td><td>382</td><td>383</td><td>384</td><td>385</td><td>386</td><td>387</td><td>388</td></tr> | ||
| + | <tr><td>L</td><td>D</td><td>M</td><td>M</td><td>G</td><td>M</td><td>Q</td><td>M</td><td>L</td><td>M</td><td>E</td><td>K</td><td>Y</td><td>G</td><td>D</td><td>Q</td><td>A</td><td>M</td><td>A</td><td>G</td><td>M</td><td>D</td><td>H</td><td>S</td><td>Q</td><td>M</td><td>M</td><td>G</td><td>H</td><td>M</td></tr> | ||
| + | |||
| + | <tr><td>389</td><td>390</td><td>391</td><td>392</td><td>393</td><td>394</td><td>395</td><td>396</td><td>397</td><td>398</td><td>399</td><td>400</td><td>401</td><td>402</td><td>403</td><td>404</td><td>405</td><td>406</td><td>407</td><td>408</td><td>409</td><td>410</td><td>411</td><td>412</td><td>413</td><td>414</td><td>415</td><td>416</td><td>417</td><td>418</td></tr> | ||
| + | <tr><td>G</td><td>H</td><td>G</td><td>N</td><td>M</td><td>N</td><td>H</td><td>M</td><td>N</td><td>H</td><td>G</td><td>G</td><td>K</td><td>F</td><td>D</td><td>F</td><td>H</td><td>H</td><td>A</td><td>N</td><td>K</td><td>I</td><td>N</td><td>G</td><td>Q</td><td>A</td><td>F</td><td>D</td><td>M</td><td>N</td></tr> | ||
| + | <tr><td>419</td><td>420</td><td>421</td><td>422</td><td>423</td><td>424</td><td>425</td><td>426</td><td>427</td><td>428</td><td>429</td><td>430</td><td>431</td><td>432</td><td>433</td><td>434</td><td>445</td><td>436</td><td>437</td><td>438</td><td>439</td><td>440</td><td>441</td><td>442</td><td class="sblockDat">443</td><td>444</td><td>445</td><td bgcolor="#999999">446</td><td>447</td><td bgcolor="#FF0000">448</td></tr> | ||
| + | <tr><td>K</td><td>P</td><td>M</td><td>F</td><td>A</td><td>A</td><td>A</td><td>K</td><td>G</td><td>Q</td><td>Y</td><td>E</td><td>R</td><td>W</td><td>V</td><td>I</td><td>S</td><td>G</td><td>V</td><td>G</td><td>D</td><td>M</td><td>M</td><td>L</td><td class="sblockDat">H</td><td>P</td><td>F</td><td bgcolor="#999999">H</td><td>I</td><td bgcolor="#FF0000">H</td> | ||
| + | <tr><td bgcolor="#CCFFFF">449</td><td>450</td><td>451</td><td>452</td><td>453</td><td>454</td><td>455</td><td>456</td><td>457</td><td>458</td><td>459</td><td>460</td><td>461</td><td>462</td><td>463</td><td>464</td><td>465</td><td>466</td><td>467</td><td>468</td><td>469</td><td>470</td><td>471</td><td>472</td><td>473</td><td>474</td><td>475</td><td>476</td><td>477</td><td>478</td></tr> | ||
| + | <tr><td bgcolor="#CCFFFF">G</td><td>T</td><td>Q</td><td>F</td><td>R</td><td>I</td><td>L</td><td>S</td><td>E</td><td>N</td><td>G</td><td>K</td><td>P</td><td>P</td><td>A</td><td>A</td><td>H</td><td>R</td><td>A</td><td>G</td><td>W</td><td>K</td><td>D</td><td>T</td><td>V</td><td>K</td><td>V</td><td>E</td><td>G</td><td>N</td> | ||
| + | <tr><td>479</td><td>480</td><td>481</td><td>482</td><td>483</td><td>484</td><td>485</td><td>486</td><td>487</td><td>488</td><td>489</td><td>490</td><td>491</td><td>492</td><td>493</td><td>494</td><td>495</td><td>496</td><td>497</td><td>498</td><td bgcolor="#FF0000">499</td><td class="sblockDat">500</td><td class="sblockLbl">501</td><td class="sblockDat">502</td><td>503</td><td>504</td><td class="sblockDat">505</td><td>506</td><td>507</td><td>508</td></tr> | ||
| + | <tr><td>V</td><td>S</td><td>E</td><td>V</td><td>L</td><td>V</td><td>K</td><td>F</td><td>N</td><td>H</td><td>D</td><td>A</td><td>P</td><td>K</td><td>E</td><td>H</td><td>A</td><td>Y</td><td>M</td><td>A</td><td bgcolor="#FF0000">H</td><td class="sblockDat">C</td><td class="sblockLbl">H</td><td class="sblockDat">L</td><td>L</td><td>E</td><td class="sblockDat">H</td><td>E</td><td>D</td><td>T</td> | ||
| + | <tr><td>509</td><td class="sblockDat">510</td><td>511</td><td>512</td><td>513</td><td>514</td><td>515</td><td>516</td></tr> | ||
| + | <tr><td>G</td><td class="sblockDat">M</td><td>M</td><td>L</td><td>G</td><td>F</td><td>T</td><td>V</td></tr> | ||
| + | <tr><td colspan='4' class="sblockDat">Stabilisation of Cu1001 <br> | ||
| + | </td></tr> | ||
| + | <tr><td colspan='4' class="sblockLbl">Stabilisation of Cu1002 <br> | ||
| + | </td></tr> | ||
| + | <tr><td colspan='4' bgcolor="#FF0000">Stabilisation of Cu1003 <br> | ||
| + | </td></tr> | ||
| + | <tr><td colspan='4' bgcolor="#999999">Stabilisation of Cu1004 <br> | ||
| + | </td></tr> | ||
| + | <tr><td colspan='4' bgcolor="#CCFFFF">Stabilisation of Act1005 <br> | ||
| + | </td></tr> | ||
| + | </table><ref>PMID:17804014</ref> | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
| + | <div style="background-color:#fffaf0;"> | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Multicopper Oxidase CueO (4e9s)
| |||||||||||
References
- ↑ EMBL-EBI, Family: Cu-oxidase (PF00394), Summary: Multicopper oxidase, http://pfam.xfam.org/family/Cu-oxidase
- ↑ UniProtKB
- ↑ Messerschmidt A, Huber R, The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships, Eur. J. Biochem. 187, January 1990
- ↑ Ouzounis C, Sander C, A structure-derived sequence pattern for the detection of type I copper binding domains in distantly related proteins, FEBS Lett. volume 279, February 1991
- ↑ Hirofumi Komori, Ryosuke Sugiyama, Kunishige Kataoka, Kentaro Miyazaki, Yoshiki Higuchib, and Takeshi Sakurai, New insights into the catalytic active-site structure of multicopper oxidases, Biological Crystallography, 6 December 2013 doi:10.1107/S1399004713033051
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ RCBS PDB
- ↑ Kataoka K, Komori H, Ueki Y, Konno Y, Kamitaka Y, Kurose S, Tsujimura S, Higuchi Y, Kano K, Seo D, Sakurai T. Structure and function of the engineered multicopper oxidase CueO from Escherichia coli--deletion of the methionine-rich helical region covering the substrate-binding site. J Mol Biol. 2007 Oct 12;373(1):141-52. Epub 2007 Aug 2. PMID:17804014 doi:10.1016/j.jmb.2007.07.041