Sandbox Reserved 1489
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
=='''2JLN'''== | =='''2JLN'''== | ||
<StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | 2JLN is one of the conformations of Mhp1, which is a membrane secondary transporter.<ref>PMID 18927357</ref> | + | 2JLN is one of the conformations of Mhp1, which is a membrane secondary transporter. |
| + | The main information of this Proteopedia page come from the article : Structure and Molecular Mechanism of a Nucleobase-Cation-Symport-1 Family Transporter.<ref>PMID 18927357</ref> | ||
== '''Function''' == | == '''Function''' == | ||
| Line 8: | Line 9: | ||
Mhp1 is a trans-membrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. | Mhp1 is a trans-membrane protein bellowing to the nucleobase-cation-symport-1 (NCS1) transporter family from Microbacterium liquefaciens. | ||
| - | A secondary transporter, like Mhp1, effects the cellular uptake and release of a wide range of substances across biological membranes in all organisms. This is done by coupling the uphill movement of the substrate against its concentration gradient with the energetically favorable downhill gradient of a second substrate, often a proton or a cation. The kinetics and thermodynamics of the transporters can be explained by their alternating conformations.<ref name=" | + | A secondary transporter, like Mhp1, effects the cellular uptake and release of a wide range of substances across biological membranes in all organisms. This is done by coupling the uphill movement of the substrate against its concentration gradient with the energetically favorable downhill gradient of a second substrate, often a proton or a cation. The kinetics and thermodynamics of the transporters can be explained by their alternating conformations.<ref name="art2">PMID 20413494</ref> |
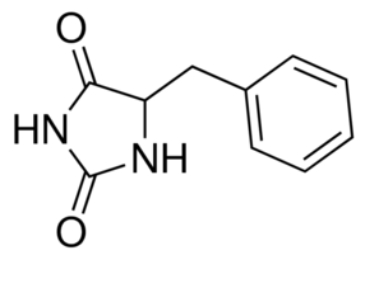
In the case of Mhp1, it allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins (''Figure 1'') in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | In the case of Mhp1, it allows the sodium dependent income of indolyl methyl- and benzyl-hydantoins (''Figure 1'') in the cell. Those are part of a salvage metabolic pathway leading to their conversion in amino acids. | ||
| Line 31: | Line 32: | ||
=='''Disease'''== | =='''Disease'''== | ||
| - | Dysfunction of members of the transporters family in humans can lead to diseases including neurological and kidney disorders. Other members are implicated in cancer as they can supply tumor cells with nutrients, cause drug resistance and/or provide a means of treatment.<ref name=" | + | Dysfunction of members of the transporters family in humans can lead to diseases including neurological and kidney disorders. Other members are implicated in cancer as they can supply tumor cells with nutrients, cause drug resistance and/or provide a means of treatment.<ref name="art2" /> |
| Line 47: | Line 48: | ||
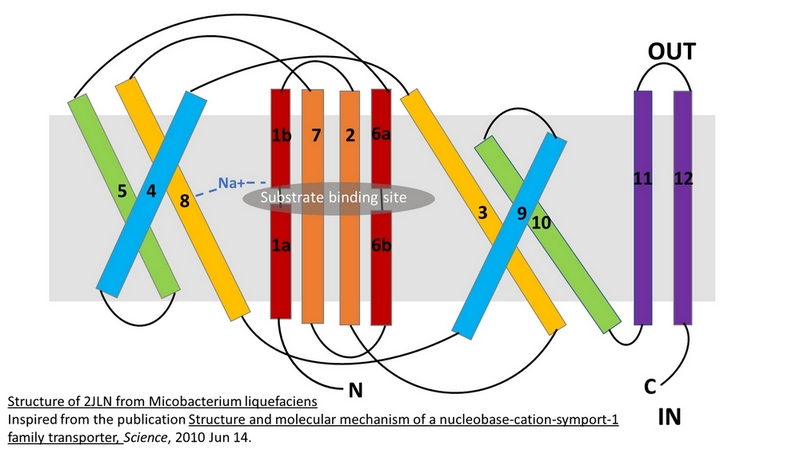
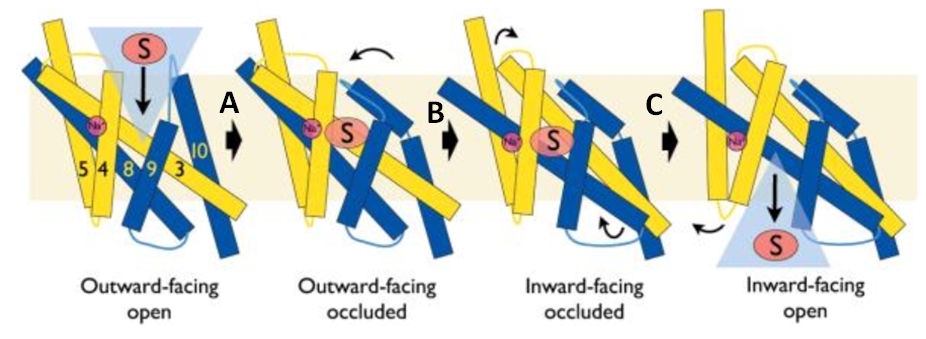
The central bundle is composed of TMs 1 and 2, twined to the TMs 6 and 7 respectively. In addition, the protein presents a V-shape structure formed by TMs 3 to 5, twined to TMs 8 to 10 (''Figure 2''). | The central bundle is composed of TMs 1 and 2, twined to the TMs 6 and 7 respectively. In addition, the protein presents a V-shape structure formed by TMs 3 to 5, twined to TMs 8 to 10 (''Figure 2''). | ||
| - | TM5 and TM10 are 'flexible helices' because they bend during the state transitions.<ref | + | TM5 and TM10 are 'flexible helices' because they bend during the state transitions.<ref name="art2" /> |
The substrate- and cation-binding sites are located in the space between the central four-helix-bundle and the outer helix layer. | The substrate- and cation-binding sites are located in the space between the central four-helix-bundle and the outer helix layer. | ||
| Line 70: | Line 71: | ||
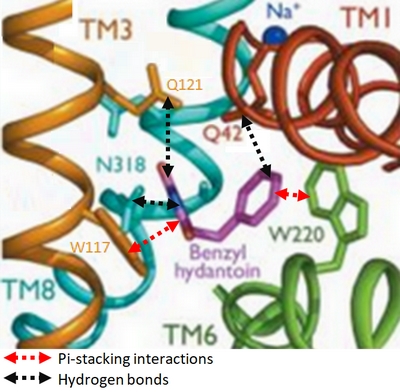
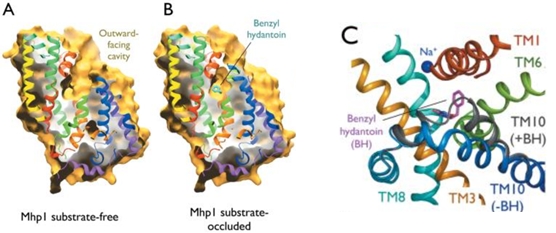
Experiments have shown that sodium increases the affinity of benzyl-hydantoin for Mhp1 and reciprocally benzyl-hydantoin increases the affinity of sodium for Mhp1. | Experiments have shown that sodium increases the affinity of benzyl-hydantoin for Mhp1 and reciprocally benzyl-hydantoin increases the affinity of sodium for Mhp1. | ||
| - | Indeed, the presence of benzyl-hydantoin in Mhp1 binding site blocks the pathway of the sodium ion to the extracellular side.<ref | + | Indeed, the presence of benzyl-hydantoin in Mhp1 binding site blocks the pathway of the sodium ion to the extracellular side.<ref name="art2" /> |
Therefore, the binding of the substrate and the cation are closely coupled. | Therefore, the binding of the substrate and the cation are closely coupled. | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
2JLN
| |||||||||||
References
- ↑ Weyand S, Shimamura T, Yajima S, Suzuki S, Mirza O, Krusong K, Carpenter EP, Rutherford NG, Hadden JM, O'Reilly J, Ma P, Saidijam M, Patching SG, Hope RJ, Norbertczak HT, Roach PC, Iwata S, Henderson PJ, Cameron AD. Structure and Molecular Mechanism of a Nucleobase-Cation-Symport-1 Family Transporter. Science. 2008 Oct 16. PMID:18927357
- ↑ 2.0 2.1 2.2 2.3 Shimamura T, Weyand S, Beckstein O, Rutherford NG, Hadden JM, Sharples D, Sansom MS, Iwata S, Henderson PJ, Cameron AD. Molecular basis of alternating access membrane transport by the sodium-hydantoin transporter Mhp1. Science. 2010 Apr 23;328(5977):470-3. PMID:20413494 doi:328/5977/470
- ↑ Simmons KJ, Jackson SM, Brueckner F, Patching SG, Beckstein O, Ivanova E, Geng T, Weyand S, Drew D, Lanigan J, Sharples DJ, Sansom MS, Iwata S, Fishwick CW, Johnson AP, Cameron AD, Henderson PJ. Molecular mechanism of ligand recognition by membrane transport protein, Mhp1. EMBO J. 2014 Jun 21. pii: e201387557. PMID:24952894 doi:http://dx.doi.org/10.15252/embj.201387557