Sandbox Reserved 1502
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
<StructureSection load='3lpt' size='340' side='right' caption='[[3lpt]], [[Resolution|resolution]] 2.00Å' scene=''> | <StructureSection load='3lpt' size='340' side='right' caption='[[3lpt]], [[Resolution|resolution]] 2.00Å' scene=''> | ||
| - | + | The 3lpt is an integrase of the HIV, the Human Immunodeficiency Virus. An integrase is an enzyme produced by a retrovirus to integrate its genetic material into the DNA of the infected cell. It is one of three enzymes of HIV, the others being the Reverse Transcriptase and the Protease. | |
== Structural highlights == | == Structural highlights == | ||
| Line 31: | Line 31: | ||
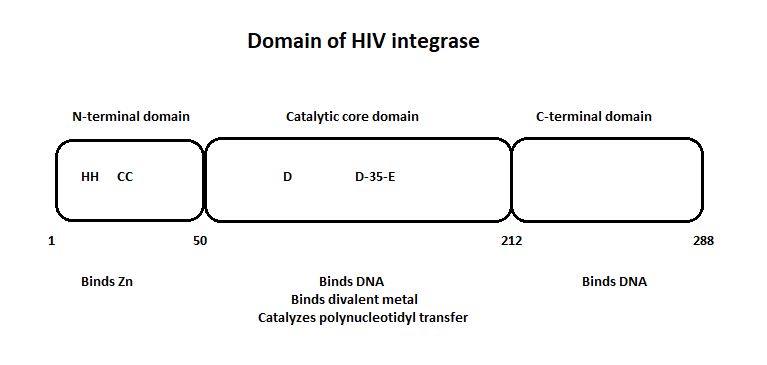
'''The three domain of the HIV integrase:''' | '''The three domain of the HIV integrase:''' | ||
| - | [[Image:Domain_HIV_Integrase.jpg]] | + | [[Image:Domain_HIV_Integrase.jpg]] [http://www.jbc.org/content/276/26/23213.full] |
'''HIV Integrase sequence:''' | '''HIV Integrase sequence:''' | ||
| Line 38: | Line 38: | ||
Length:288, Mass (Da):32,199 | Length:288, Mass (Da):32,199 | ||
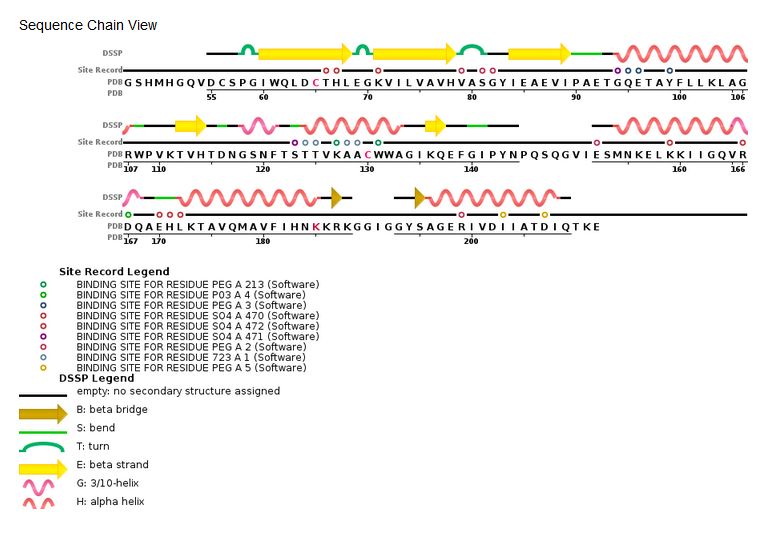
| - | '''Sequence | + | '''Sequence chain view of the HIV Integrase:''' |
[[Image:HIV Integrase sequence.jpg]] [http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=3lpt] | [[Image:HIV Integrase sequence.jpg]] [http://www.rcsb.org/pdb/explore/remediatedSequence.do?structureId=3lpt] | ||
| Line 44: | Line 44: | ||
====Structure and role of the core domain==== | ====Structure and role of the core domain==== | ||
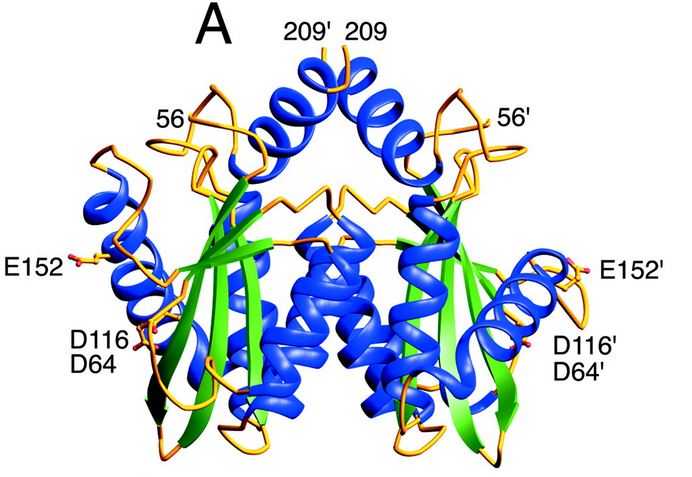
| - | The catalytic core domain contains three acidic residues, the D,D-35-E motif, comprising residues Asp64, Asp116, and Glu152. By analogy with models of catalysis by DNA polymerases, it has been proposed that coordination of divalent metal ion to these residues plays a key role in catalysis. The catalytic residues Asp64, Asp116, and | + | The catalytic core domain contains three acidic residues, the D,D-35-E motif, comprising residues Asp64, Asp116, and Glu152. By analogy with models of catalysis by DNA polymerases, it has been proposed that coordination of divalent metal ion to these residues plays a key role in catalysis. The catalytic residues Asp64, Asp116, and Glu152 of HIV integrase are in close proximity, coordinate divalent metal ion, and define the active site. The residues comprising the active site region exhibit considerable flexibility. That suggests that binding of DNA substrate is required to impose the precise configuration of residues that are required for catalysis. |
| - | [[Image:Core enzyme.jpg]] | + | [[Image:Core enzyme.jpg]] [http://www.jbc.org/content/276/26/23213.full] |
<scene name='80/802676/Core_enzyme/2'>Here is the 3D structure of the core domain</scene> | <scene name='80/802676/Core_enzyme/2'>Here is the 3D structure of the core domain</scene> | ||
| Line 53: | Line 53: | ||
====Structure and role of the N ter domain==== | ====Structure and role of the N ter domain==== | ||
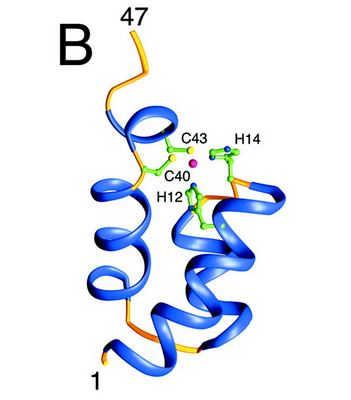
| - | The N-terminal domain of HIV-1 integrase contains a conserved pair of His and Cys residues, a motif similar to the zinc-coordinating residues of zinc fingers. This domain binds zinc but its structure is totally different from that of zinc fingers. It consists of a bundle of three α-helices. | + | The N-terminal domain of HIV-1 integrase contains a conserved pair of His and Cys residues, a motif similar to the zinc-coordinating residues of zinc fingers. This domain binds zinc but its structure is totally different from that of zinc fingers. It consists of a bundle of three α-helices. This domain is necessary for the oligomerization of the integrase. |
| - | [[Image:Nter-domain.jpg]] | + | [[Image:Nter-domain.jpg]] [http://www.jbc.org/content/276/26/23213.full] |
<scene name='80/802676/N-ter_domain/2'>Here is the 3D structure of the N-ter domain of the HIV integrase</scene> | <scene name='80/802676/N-ter_domain/2'>Here is the 3D structure of the N-ter domain of the HIV integrase</scene> | ||
| Line 63: | Line 63: | ||
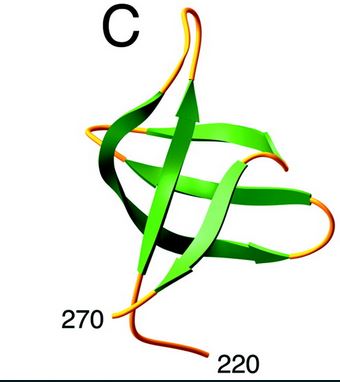
====Structure and role of the C ter domain==== | ====Structure and role of the C ter domain==== | ||
| - | [[Image:C-ter domain.jpg]] | + | Here is the structure of the C-ter domain : |
| + | |||
| + | [[Image:C-ter domain.jpg]] [http://www.jbc.org/content/276/26/23213.full] | ||
| + | |||
| + | The C-terminal domain interacts in a non-specific way with DNA and would, therefore, play a stabilizing role of integrase-ADN interactions. | ||
| Line 72: | Line 76: | ||
====Use of antiviral to inhibit the LEDGF/p75-integrase interaction ==== | ====Use of antiviral to inhibit the LEDGF/p75-integrase interaction ==== | ||
| - | An antiviral refers to a molecule disrupting the replication cycle of one or more viruses, thus allowing to slow down but rarely to stop a viral infection. Antiviral targeting specific regions of the integrase are used to prevent the replication of HIV. For example, the | + | An antiviral refers to a molecule disrupting the replication cycle of one or more viruses, thus allowing to slow down but rarely to stop a viral infection. Antiviral targeting specific regions of the integrase are used to prevent the replication of HIV. For example, the 2-(quinolin-3-yl)acetic acid interacts with <scene name='80/802676/Site_of_interaction/7'>the binding site of the LEDGF/p75 cofactor</scene>. More precisely, it binds to the residues E170, H171, and T174. This site is situated in the core domain of the enzyme. Once it is fixed into the core domain, the LEDGF/p75 will not be able to join and viral integration of the retrovirus will not be possible anymore. |
The whole complex between the integrase and the cofactor is displayed on the Proteopedia page [[2b4j]]. | The whole complex between the integrase and the cofactor is displayed on the Proteopedia page [[2b4j]]. | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
3lpt - HIV integrase
| |||||||||||