Sandbox Reserved 1490
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
It acts as cell-surface receptor for the ligands ANGPT1, ANGPT2 and ANGPT4 and regulates among others angiogenesis, endothelial cell survival and maintenance of vascular quiescence. It is important in the regulation of both normal physiologic and pathologic angiogenesis. The later is a fundamental step in the transition of tumors from a benign state to a malignant one. | It acts as cell-surface receptor for the ligands ANGPT1, ANGPT2 and ANGPT4 and regulates among others angiogenesis, endothelial cell survival and maintenance of vascular quiescence. It is important in the regulation of both normal physiologic and pathologic angiogenesis. The later is a fundamental step in the transition of tumors from a benign state to a malignant one. | ||
| - | + | Angiogenesis is the process in which new blood vessels are formed from pre-existing blood vessels. The growth of these new blood vessels requires migration and proliferation of endothelial cells (ECs). It is an event controlled by angiogenic growth factors such as vascular endothelial growth factor (VEGF). | |
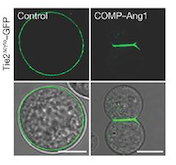
While ANGPT1 is a TIE2 agonist and has a higher binding affinity to it than ANGPT2, ANGPT2 can act as a context-dependent agonist. Thus, the ANGPT/TIE2 kinase signaling pathway is an attractive anti-vascular target. | While ANGPT1 is a TIE2 agonist and has a higher binding affinity to it than ANGPT2, ANGPT2 can act as a context-dependent agonist. Thus, the ANGPT/TIE2 kinase signaling pathway is an attractive anti-vascular target. | ||
| Line 44: | Line 44: | ||
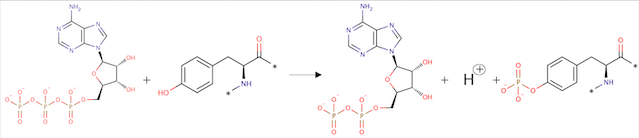
Angiopoietin binding leads to receptor dimerization and activation by autophosphorylation at Tyr-992 on the kinase activation loop. | Angiopoietin binding leads to receptor dimerization and activation by autophosphorylation at Tyr-992 on the kinase activation loop. | ||
[[Image:Réaction Phosphorylation.jpg]] | [[Image:Réaction Phosphorylation.jpg]] | ||
| + | |||
''Fig 2. Phosphorylation reaction'' | ''Fig 2. Phosphorylation reaction'' | ||
| Line 49: | Line 50: | ||
===• Description of total protein=== | ===• Description of total protein=== | ||
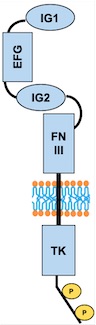
[[Image:TIE2 Schema.jpg]] | [[Image:TIE2 Schema.jpg]] | ||
| + | |||
''Fig 3. Scheme of the whole TIE2 receptor'' | ''Fig 3. Scheme of the whole TIE2 receptor'' | ||
====Important sites ==== | ====Important sites ==== | ||
| Line 264: | Line 266: | ||
== Medical relevance == | == Medical relevance == | ||
===•Venous Malformations=== | ===•Venous Malformations=== | ||
| - | Venous malformations can cause significant morbidity due to pain, disfigurement and organ dysfunction. Before understanding a lot better the mechanisms leading to this disease, therapies were limited to compression therapy and ablation of malformed veins by sclerotherapy and surgery.<ref>PMID:29668117</ref> | + | Venous malformations can cause significant morbidity due to pain, disfigurement and organ dysfunction. Before understanding a lot better the mechanisms leading to this disease, therapies were limited to compression therapy and ablation of malformed veins by sclerotherapy and surgery.<ref name="Therapies for Venous Malformations">PMID: 29668117</ref> |
A gene test for TIE2 and PIK3CA mutations is the most definite biomarker for VMs. The mutations in the sequence of this proteins cover a large proportion of the causes (about 80%) of all VMs. | A gene test for TIE2 and PIK3CA mutations is the most definite biomarker for VMs. The mutations in the sequence of this proteins cover a large proportion of the causes (about 80%) of all VMs. | ||
In a blood coagulation reaction, fibrinogen is transformed to fibrin that is cleaved by plasmin in fibrinolysis, resulting in the formation of D‐dimers as a fibrin degradation product. | In a blood coagulation reaction, fibrinogen is transformed to fibrin that is cleaved by plasmin in fibrinolysis, resulting in the formation of D‐dimers as a fibrin degradation product. | ||
| - | Unlike other vascular malformations, VMs patients often have elevated D‐dimers. D‐dimer testing has shown to be useful to separate VMs from other vascular or lymphatic malformations which usually present with normal D‐dimers. Interestingly, VM patients with identified TIE2 or PIK3CA mutations had high D‐dimers when compared to patients with no detectable mutation in these genes. A high serum level of D‐dimers is not solely due to static blood flow in the lesions, but also to an intrinsic signalling defect in ECs due to constantly high TIE2/PIK3CA activity.<ref | + | Unlike other vascular malformations, VMs patients often have elevated D‐dimers. D‐dimer testing has shown to be useful to separate VMs from other vascular or lymphatic malformations which usually present with normal D‐dimers. Interestingly, VM patients with identified TIE2 or PIK3CA mutations had high D‐dimers when compared to patients with no detectable mutation in these genes. A high serum level of D‐dimers is not solely due to static blood flow in the lesions, but also to an intrinsic signalling defect in ECs due to constantly high TIE2/PIK3CA activity.<ref name="Therapies for Venous Malformations"/> |
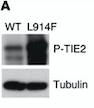
[[Image:electro.png]] | [[Image:electro.png]] | ||
| - | ''Fig 6. Western blot of p-TIE2 in Human Endothelial Cells transfected with TIE2-WT (Wild type) or with mutant TIE2 (L914F). Tubulin served as loading control. The hyperphosphorylation is clearly visible.''<ref>PMID:26258417</ref> | + | ''Fig 6. Western blot of p-TIE2 in Human Endothelial Cells transfected with TIE2-WT (Wild type) or with mutant TIE2 (L914F). Tubulin served as loading control. The hyperphosphorylation is clearly visible.''<ref name="Molecular Therapies">PMID: 26258417</ref> |
Thus, genetic and transplantation‐based models offer versatile tools to study the pathology of VMs, as well as the efficacy and safety of potential molecular therapies. | Thus, genetic and transplantation‐based models offer versatile tools to study the pathology of VMs, as well as the efficacy and safety of potential molecular therapies. | ||
| - | Rapamycin is the first molecular therapy for VMs. It is currently being tested in a multicenter clinical trial on lymphatico-vascular malformations.<ref | + | Rapamycin is the first molecular therapy for VMs. It is currently being tested in a multicenter clinical trial on lymphatico-vascular malformations.<ref name="Molecular Therapies"/> |
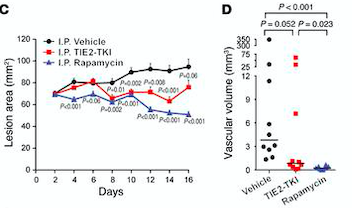
[[Image:lesion area.png]] | [[Image:lesion area.png]] | ||
| - | ''Fig 7. (C) HUVECs lesional area measured every 2 days for 16 days. (D) Vascular volume at day 15 measured by analysis of color Doppler 3D image stacks. When compared with the vehicle-treated group, the lesional area was significantly smaller in the rapamycin-treated group from day 4 to day 16 and in the TIE2-TKI–treated group from day 8 to day 14.''<ref | + | ''Fig 7. (C) HUVECs lesional area measured every 2 days for 16 days. (D) Vascular volume at day 15 measured by analysis of color Doppler 3D image stacks. When compared with the vehicle-treated group, the lesional area was significantly smaller in the rapamycin-treated group from day 4 to day 16 and in the TIE2-TKI–treated group from day 8 to day 14.''<ref name="Molecular Therapies"/> |
===•Cancers=== | ===•Cancers=== | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
Crystal structure of cytoplasmic kinase domain of Tie2 in complex with decipera compound DP1919
| |||||||||||
References
- ↑ Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008 May;10(5):513-26. doi: 10.1038/ncb1714. Epub 2008 Apr 20. PMID:18425120 doi:10.1038/ncb1714
- ↑ 2.0 2.1 Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009 Apr;29(8):2011-22. doi: 10.1128/MCB.01472-08. Epub 2009 Feb, 17. PMID:19223473 doi:10.1128/MCB.01472-08
- ↑ 3.0 3.1 Murray BW, Padrique ES, Pinko C, McTigue MA. Mechanistic effects of autophosphorylation on receptor tyrosine kinase catalysis: enzymatic characterization of Tie2 and phospho-Tie2. Biochemistry. 2001 Aug 28;40(34):10243-53. PMID:11513602
- ↑ 4.0 4.1 4.2 Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. A unique autophosphorylation site on Tie2/Tek mediates Dok-R phosphotyrosine binding domain binding and function. Mol Cell Biol. 2003 Apr;23(8):2658-68. PMID:12665569
- ↑ 5.0 5.1 5.2 Vikkula M, Boon LM, Carraway KL 3rd, Calvert JT, Diamonti AJ, Goumnerov B, Pasyk KA, Marchuk DA, Warman ML, Cantley LC, Mulliken JB, Olsen BR. Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell. 1996 Dec 27;87(7):1181-90. PMID:8980225
- ↑ 6.0 6.1 Kangas J, Natynki M, Eklund L. Development of Molecular Therapies for Venous Malformations. Basic Clin Pharmacol Toxicol. 2018 Sep;123 Suppl 5:6-19. doi: 10.1111/bcpt.13027., Epub 2018 May 29. PMID:29668117 doi:http://dx.doi.org/10.1111/bcpt.13027
- ↑ 7.0 7.1 7.2 Boscolo E, Limaye N, Huang L, Kang KT, Soblet J, Uebelhoer M, Mendola A, Natynki M, Seront E, Dupont S, Hammer J, Legrand C, Brugnara C, Eklund L, Vikkula M, Bischoff J, Boon LM. Rapamycin improves TIE2-mutated venous malformation in murine model and human subjects. J Clin Invest. 2015 Sep;125(9):3491-504. doi: 10.1172/JCI76004. Epub 2015 Aug 10. PMID:26258417 doi:http://dx.doi.org/10.1172/JCI76004
- ↑ Shlamkovich T, Aharon L, Koslawsky D, Einav Y, Papo N. Targeting the Tie2-alphavbeta3 integrin axis with bi-specific reagents for the inhibition of angiogenesis. BMC Biol. 2018 Aug 17;16(1):92. doi: 10.1186/s12915-018-0557-9. PMID:30119679 doi:http://dx.doi.org/10.1186/s12915-018-0557-9