Journal:BAMBEd:Acetylcholinesterase: Substrate Traffic and Inhibition
From Proteopedia
(Difference between revisions)

| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
<StructureSection load='2ace' size='350' side='right' scene='Sandbox_250/Ache_ach/30' caption=''> | <StructureSection load='2ace' size='350' side='right' scene='Sandbox_250/Ache_ach/30' caption=''> | ||
| - | + | ===Acetylcholinesterase: Substrate traffic and inhibition=== | |
| + | <big>Mary G. Acheampong, Daviana E. Dueño, Bobby K. Glover, Alafia A. Henry, Randol Mata, Marisa L. VanBrakle, Lars F. Westblade, Joel L. Sussman, Allison L. Granberry</big> <ref>doi: 10.1002/bmb.20604</ref> | ||
| + | <hr/> | ||
=='''Introduction'''== | =='''Introduction'''== | ||
| - | |||
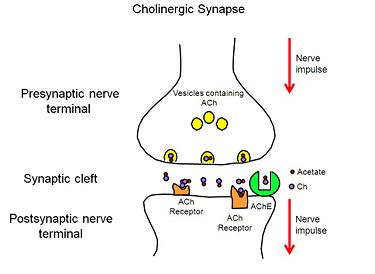
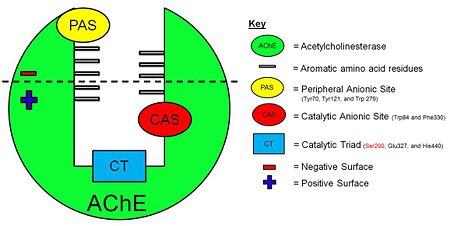
[[Acetylcholinesterase]] (AChE) is essential for hydrolysis of the neurotransmitter acetylcholine (ACh), and, therefore, for termination of impulse transmission at cholinergic synapses (Figure 2). Irreversible inhibition of AChE can result in accumulation of ACh at cholinergic synapses and, ultimately, to death. Conversely, decreased levels of ACh may result in the memory deficits associated with Alzheimer's disease<ref>PMID: 14501022</ref>. AChE has a deep (20Å) and narrow (5Å) gorge lined with 14 aromatic residues, with its active site located near the bottom of the gorge<ref>PMID: 1678899</ref>. Initially, ACh binds to the peripheral anionic site (PAS) of AChE, and is funneled down the gorge to the active site by interactions between its quaternary ammonium group and the aromatic rings of 14 aromatic amino acid residues lining the gorge. At the active site, ACh is oriented for hydrolysis by interactions between the catalytic anionic site and its quaternary ammonium group. Fasciculin-II (FAS-II), a potent polypeptide toxin present in the venom of the East African green mamba (Dendroaspis angusticeps), inhibits AChE by binding to the top of the active-site gorge, interacting tightly with residues that form the PAS; it thus prevents ACh from entering the active-site gorge<ref>PMID:8747462</ref>. The Hostos-Lincoln Academy Students Modeling A Research Topic (S.M.A.R.T) team and the Center for BioMolecular Modeling have designed and fabricated two physical models using a combination of computational molecular modeling and three-dimensional (3D) printing technology: ''Torpedo californica'' (''Tc'') AChE complexed with a modeled ACh molecule ligand, and a complex of FAS-II with ''Tc''AChE. | [[Acetylcholinesterase]] (AChE) is essential for hydrolysis of the neurotransmitter acetylcholine (ACh), and, therefore, for termination of impulse transmission at cholinergic synapses (Figure 2). Irreversible inhibition of AChE can result in accumulation of ACh at cholinergic synapses and, ultimately, to death. Conversely, decreased levels of ACh may result in the memory deficits associated with Alzheimer's disease<ref>PMID: 14501022</ref>. AChE has a deep (20Å) and narrow (5Å) gorge lined with 14 aromatic residues, with its active site located near the bottom of the gorge<ref>PMID: 1678899</ref>. Initially, ACh binds to the peripheral anionic site (PAS) of AChE, and is funneled down the gorge to the active site by interactions between its quaternary ammonium group and the aromatic rings of 14 aromatic amino acid residues lining the gorge. At the active site, ACh is oriented for hydrolysis by interactions between the catalytic anionic site and its quaternary ammonium group. Fasciculin-II (FAS-II), a potent polypeptide toxin present in the venom of the East African green mamba (Dendroaspis angusticeps), inhibits AChE by binding to the top of the active-site gorge, interacting tightly with residues that form the PAS; it thus prevents ACh from entering the active-site gorge<ref>PMID:8747462</ref>. The Hostos-Lincoln Academy Students Modeling A Research Topic (S.M.A.R.T) team and the Center for BioMolecular Modeling have designed and fabricated two physical models using a combination of computational molecular modeling and three-dimensional (3D) printing technology: ''Torpedo californica'' (''Tc'') AChE complexed with a modeled ACh molecule ligand, and a complex of FAS-II with ''Tc''AChE. | ||
=='''Background Information'''== | =='''Background Information'''== | ||
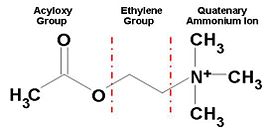
| - | [[Image:AChE-Page-ACh_shematic.JPG|thumb|alt= Alt text| Figure 1. Chemical Structure of Acetylcholine |275px]] | + | [[Image:AChE-Page-ACh_shematic.JPG|center|thumb|alt= Alt text| Figure 1. Chemical Structure of Acetylcholine |275px]] |
{{clear}} | {{clear}} | ||
When a nerve impulse reaches the presynaptic nerve terminal of a cholinergic synpase, it stimulates the release of the neurotransmitter, ACh (Figure 1), into the synaptic cleft. ACh diffuses across the cleft to the postsynaptic nerve terminal, where it binds reversibly to acetylcholine receptors embedded in the membrane of the postsynaptic nerve terminal. The binding of ACh to the receptors triggers a nerve impulse in the postsynaptic neuron. Finally AChE, anchored to the membrane of the postsynaptic nerve terminal (Figure 2), hydrolyzes ACh to acetate and choline, resulting in the termination of neurotransmission. | When a nerve impulse reaches the presynaptic nerve terminal of a cholinergic synpase, it stimulates the release of the neurotransmitter, ACh (Figure 1), into the synaptic cleft. ACh diffuses across the cleft to the postsynaptic nerve terminal, where it binds reversibly to acetylcholine receptors embedded in the membrane of the postsynaptic nerve terminal. The binding of ACh to the receptors triggers a nerve impulse in the postsynaptic neuron. Finally AChE, anchored to the membrane of the postsynaptic nerve terminal (Figure 2), hydrolyzes ACh to acetate and choline, resulting in the termination of neurotransmission. | ||
| - | [[Image:AChE-Page-Cholinergic-Synapse.jpg| | + | [[Image:AChE-Page-Cholinergic-Synapse.jpg|center|thumb|alt= Alt text| Figure 2. Cholinergic Synapse |375px]] |
{{clear}} | {{clear}} | ||
Inhibition of AChE may result in various outcomes, depending on the physiological context. Toxins such as FAS-II, from the green mamba, a poisonous snake found in East Africa, inhibit AChE and ultimately lead to death. However, controlled inhibition of AChE, in patients with Alzheimer’s disease, by drugs designed for this purpose, alleviates their symptoms, including memory loss and disorientation. | Inhibition of AChE may result in various outcomes, depending on the physiological context. Toxins such as FAS-II, from the green mamba, a poisonous snake found in East Africa, inhibit AChE and ultimately lead to death. However, controlled inhibition of AChE, in patients with Alzheimer’s disease, by drugs designed for this purpose, alleviates their symptoms, including memory loss and disorientation. | ||
| Line 33: | Line 34: | ||
==='''Features of the Substrate Traffic Story: ''a Model of'' AChE/ACh '''=== | ==='''Features of the Substrate Traffic Story: ''a Model of'' AChE/ACh '''=== | ||
---- | ---- | ||
| - | [[Image:AChE-Page-schematic-gorge.jpg| | + | [[Image:AChE-Page-schematic-gorge.jpg|center|thumb|alt= Alt text| Figure 3. Schematic illustration of AChE. |450px]] |
{{clear}} | {{clear}} | ||
Model of <scene name='Sandbox_250/Ache_ach/1'>AChE/ACh</scene> | Model of <scene name='Sandbox_250/Ache_ach/1'>AChE/ACh</scene> | ||
| Line 66: | Line 67: | ||
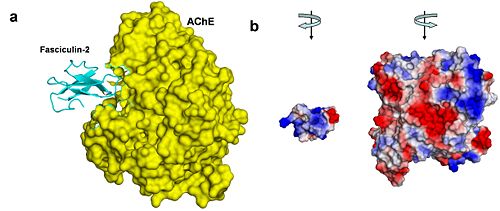
3. Shape: Once bound to the PAS, two loops of FAS-II fit in to the AChE active-site gorge like a hand fits into a glove. Once this occurs, the entrance of the gorge is <scene name='Sandbox_250/Ache_fas2/13'>blocked</scene> such that acetylcholine may not enter, and therefore it will not be hydrolysed. This results in the increased levels of AChE in the cholinergic synapse, and ultimately death. | 3. Shape: Once bound to the PAS, two loops of FAS-II fit in to the AChE active-site gorge like a hand fits into a glove. Once this occurs, the entrance of the gorge is <scene name='Sandbox_250/Ache_fas2/13'>blocked</scene> such that acetylcholine may not enter, and therefore it will not be hydrolysed. This results in the increased levels of AChE in the cholinergic synapse, and ultimately death. | ||
| - | [[Image:New_Schematic_AChE_Fas.JPG| | + | [[Image:New_Schematic_AChE_Fas.JPG|center|thumb|alt= Alt text| Figure 4. AChE-fasciculin-2 complex. (a) A side view of the complex, illustrating the geometric complementarity of the two interacting proteins. AChE is presented as a yellow surface and fasciculin-2 as a blues ribbon. (b) A front view of both interacting proteins, presented separately as surfaces colored by electrostatic potential (blue is positive, white is neutral, and red is negative). To create this view, both proteins were rotated 90º compared to their position in a, AChE to the right and fasciculin to the left. The electrostatic compatibility between the two proteins is clear; The positively charged part of fasciculin matches the entrance to AChE's binding site, which is negatively charged <ref>Kessel A and Ben-Tal N (Dec. 2010) Introduction to Proteins: Structure, Function, and Motion. Chapman & Hall/CRC Mathematical & Computational Biology. ISBN: 9781439810712</ref>.|500px]] |
{{clear}} | {{clear}} | ||
| - | + | __TOC__ | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=='''References'''== | =='''References'''== | ||
<references/> | <references/> | ||
| Line 80: | Line 76: | ||
=='''Acknowledgements'''== | =='''Acknowledgements'''== | ||
| - | |||
1. Howard Hughes Medical Institue Pre-College Program | 1. Howard Hughes Medical Institue Pre-College Program | ||
| Line 98: | Line 93: | ||
9. Malcolm Twist | 9. Malcolm Twist | ||
| - | + | ||
| - | + | __NOTOC__ | |
Current revision
| |||||||||||
Acknowledgements
1. Howard Hughes Medical Institue Pre-College Program
2. Center for BioMolecular Modeling, Milwaukee School of Engineering
3. The Rockefeller University Center for Clinical and Translational Science
4. The Rockefeller University S.M.A.R.T Team Program
5. The Rockefeller University Science Outreach Program
6. Touro College of Pharmacy
7. Michal Harel, Weizmann Institute of Science
8. Natural Sciences Department,Hostos Community College, Bronx, NY
9. Malcolm Twist
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Alexander Berchansky, Jaime Prilusky, Michal Harel, Daviana Dueno, Randol Mata, Mary Acheampong, Allison Granberry, Alafia Henry, Marisa L. VanBrakle
This page complements a publication in scientific journals and is one of the Proteopedia's Interactive 3D Complement pages. For aditional details please see I3DC.