3-phosphoinositide-dependent protein kinase 1
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
== Function == | == Function == | ||
| - | '''3-phosphoinositide-dependent protein kinase 1''' ( | + | '''3-phosphoinositide-dependent protein kinase 1''' (PDK1) activates many other kinases. PDK1 main effector is protein kinase B (Akt). <scene name='54/542348/Cv/6'>Serine (Ser241 in human) is the PDK1 phosphorylated residue (PSer)</scene>. PDK1 is important in signaling pathways activated by growth factors and hormones. It interacts with membrane phospholipids. <ref>PMID:15209375</ref> |
| + | |||
| + | PDK1 is an enzyme that plays a central role in signal transduction pathways involved in cell growth, metabolism, and survival. It belongs to the family of AGC (protein kinase A, G, and C) kinases and is a key regulator of various cellular processes. | ||
| + | |||
| + | PDK1 is known for its ability to phosphorylate and activate a group of kinases called AGC kinases, which includes protein kinase B (PKB/Akt), protein kinase C (PKC), and serum and glucocorticoid-regulated kinase (SGK), among others. PDK1 phosphorylates a conserved activation loop residue (threonine or serine) of AGC kinases, which leads to their activation and subsequent downstream signaling events. | ||
| + | |||
| + | The activation of PDK1 is tightly regulated and relies on the presence of phosphatidylinositol-3,4,5-trisphosphate (PIP3) or phosphatidylinositol-3,4-bisphosphate (PIP2), which are generated by phosphoinositide 3-kinase (PI3K) signaling. PDK1 contains a pleckstrin homology (PH) domain that specifically binds to these phosphoinositides, allowing its recruitment to the plasma membrane, where it becomes active. | ||
| + | |||
| + | Once activated, PDK1 phosphorylates the AGC kinases on a conserved residue within their activation loop, initiating a signaling cascade that regulates multiple cellular processes. These processes include cell proliferation, growth, survival, metabolism, and cytoskeletal rearrangements. | ||
| + | |||
| + | The dysregulation of PDK1 and its downstream signaling pathways has been implicated in various diseases, including cancer, diabetes, and cardiovascular disorders. Consequently, PDK1 has emerged as a potential therapeutic target for the development of novel drugs aimed at modulating these signaling pathways. | ||
| + | |||
| + | In summary, 3-phosphoinositide-dependent protein kinase 1 (PDK1) is a crucial enzyme involved in the activation of AGC kinases, which regulate a wide range of cellular processes. Its activation is dependent on phosphoinositide signaling, and it serves as a central hub in various signaling pathways involved in cell growth, metabolism, and survival. | ||
== Disease == | == Disease == | ||
| - | Inhibition of | + | Inhibition of PDK1 leads to increase in α-secretase activity at the neuronal surface causing cellular prion protein and amyloid precursor protein to be cleaved into their nonpathogenic forms. |
== Structural highlights == | == Structural highlights == | ||
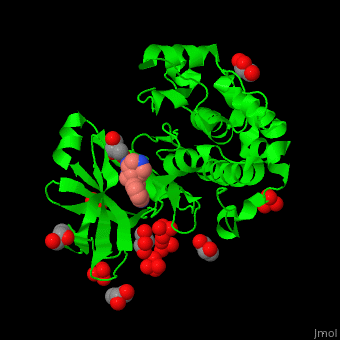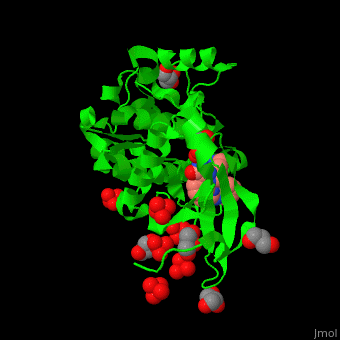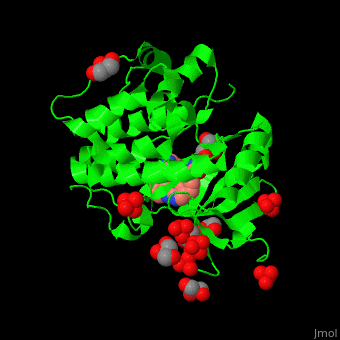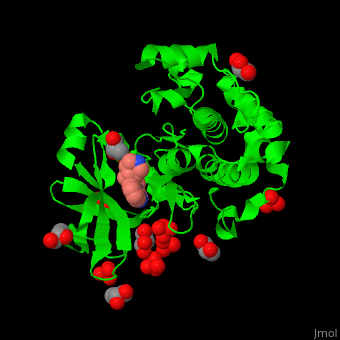
| - | + | PDK1 structure contains 2 domains: kinase (residues 71-359 in human) and PH (residues 459-550) in human). The PH (Plecksin Homology) domain interacts with phospholipids. The kinase domain (kd) contains 3 binding sites. These are for substrate-binding, ATP-binding and allosteric activator docking (PIF - [PDK1-Interacting Fragment] -pocket). <scene name='54/542348/Cv/7'>Pyrazoloquinazoline inhibitor binding site</scene>. Water molecules shown as red spheres. | |
| - | == 3D Structures of | + | == 3D Structures of PDK1== |
[[Pdk1 3D structures]] | [[Pdk1 3D structures]] | ||
</StructureSection> | </StructureSection> | ||
Current revision
| |||||||||||
References
- ↑ Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004 Apr;15(2):161-70. PMID:15209375