We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Paclitaxel
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== The Interaction of Paclitaxel with Microtubules == | == The Interaction of Paclitaxel with Microtubules == | ||
| - | <StructureSection load='1tub' size='340' side='right' caption='Paclitaxel Interacting with | + | <StructureSection load='1tub' size='340' side='right' caption='Paclitaxel Interacting with pig tubulin complex with GDP nd GTP (PDB code [[1tub]])' scene=''> |
| - | + | ||
| - | + | ||
== Function== | == Function== | ||
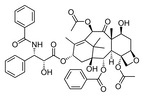
| - | Paclitaxel (also known as taxol, Figure 1) is a mitotic inhibitor used in cancer chemotherapy. It has been approved to treat ovarian, breast, and lung cancer, as well as Kaposi’s sarcoma. Paclitaxel is an antitumor drug and it plays a major role in cancer chemotherapy.<scene name='80/809872/Sami_scene_1/19'> Paclitaxel enhances the polymerization of tubulin</scene> to stable microtubules. Microtubules consist of polymers of tubulin which form part of the cytoskeleton and provide structure and shape to the cytoplasm of various cells. They are involved in cell division (by mitosis and meiosis) and are the major constituents of mitotic spindles. Paclitaxel partly induces cell death through disrupting mitosis by binding to and stabilizing the microtubule proteins. When paclitaxel binds to the microtubules, it essentially freezes them in place, preventing the separation of chromosomes during cell division. The stabilization is accompanied by structural modifications in the microtubules. The effects are different if assembly of mitotic apparatus is accompanied with the presence of paclitaxel, compared to when paclitaxel is added after the assembly. <ref name="linda">DOI: 10.1016/S1074-5521(99)89002-4</ref> | + | '''Paclitaxel''' (also known as '''taxol''', Figure 1) is a mitotic inhibitor used in cancer chemotherapy. It has been approved to treat ovarian, breast, and lung cancer, as well as Kaposi’s sarcoma. Paclitaxel is an antitumor drug and it plays a major role in cancer chemotherapy.<scene name='80/809872/Sami_scene_1/19'> Paclitaxel enhances the polymerization of tubulin</scene> to stable microtubules. Microtubules consist of polymers of tubulin which form part of the cytoskeleton and provide structure and shape to the cytoplasm of various cells. They are involved in cell division (by mitosis and meiosis) and are the major constituents of mitotic spindles. Paclitaxel partly induces cell death through disrupting mitosis by binding to and stabilizing the microtubule proteins. When paclitaxel binds to the microtubules, it essentially freezes them in place, preventing the separation of chromosomes during cell division. The stabilization is accompanied by structural modifications in the microtubules. The effects are different if assembly of mitotic apparatus is accompanied with the presence of paclitaxel, compared to when paclitaxel is added after the assembly. <ref name="linda">DOI: 10.1016/S1074-5521(99)89002-4</ref> See also [[Cancer]]. |
==History== | ==History== | ||
| Line 17: | Line 15: | ||
==Reactions and Mechanism== | ==Reactions and Mechanism== | ||
Paclitaxel has a specific binding site on the microtubule polymer, and this makes it different and more effective than other chemotherapeutic agents. It has the ability to polymerize tubulin in the absence of cofactors, which is unusual and unique. Paclitaxel binds to cells, and <scene name='80/809872/Sami_scene_12/5'>(Paclitaxel) interacts with α and β Tubulin </scene> in a specific and saturable manner and then blocks cells in the G1/M phase of the cell cycle by stabilizing the microtubule cytoskeleton against depolymerization. These cells are then unable to form normal mitotic apparatus, and eventually die. The mechanism of stabilization is not known, however, there has been strong research in support of the following mechanism. | Paclitaxel has a specific binding site on the microtubule polymer, and this makes it different and more effective than other chemotherapeutic agents. It has the ability to polymerize tubulin in the absence of cofactors, which is unusual and unique. Paclitaxel binds to cells, and <scene name='80/809872/Sami_scene_12/5'>(Paclitaxel) interacts with α and β Tubulin </scene> in a specific and saturable manner and then blocks cells in the G1/M phase of the cell cycle by stabilizing the microtubule cytoskeleton against depolymerization. These cells are then unable to form normal mitotic apparatus, and eventually die. The mechanism of stabilization is not known, however, there has been strong research in support of the following mechanism. | ||
| - | Paclitaxel binds into a pocket in the second globular domain of β- tubulin, facing the central hole in a microtubule. The corresponding space in α- tubulin is occupied by an eight-residue insertion in the loop between β- strands S9 and S10. Paclitaxel also makes close contact with the shorter S9-S10 loop in β-tubulin. The molecule also appears to touch the core helix and approach the loop between S7 and H9, now known as the M loop. When <scene name='80/809872/Sami_scene_9/5'>Paclitaxel binds to the M loop</scene>, the taxane ring (from the Paclitaxel) associates with the M group. It is not constrained by the dimer structure; however, it becomes strongly immobilized after the polymerization of tubulin. The amino acids that interact with the α- and β- tubulin are slightly different depending on the assembled isotypes. The amino acids that make up the <scene name='80/809872/Sami_scene_8/3'>binding site on β-tubulin</scene> include Asp226, His229, Val23, Arg369, Gly370, Thr276 and Arg278. The strong interaction allows for the binding of <scene name='80/809872/Sami_scene_9/6'>Paclitaxel to β tubulin</scene>. The amino acids involved with the binding of Paclitaxel to the <scene name='80/809872/Sami_scene_10/ | + | Paclitaxel binds into a pocket in the second globular domain of β- tubulin, facing the central hole in a microtubule. The corresponding space in α- tubulin is occupied by an eight-residue insertion in the loop between β- strands S9 and S10. Paclitaxel also makes close contact with the shorter S9-S10 loop in β-tubulin. The molecule also appears to touch the core helix and approach the loop between S7 and H9, now known as the M loop. When <scene name='80/809872/Sami_scene_9/5'>Paclitaxel binds to the M loop</scene>, the taxane ring (from the Paclitaxel) associates with the M group. It is not constrained by the dimer structure; however, it becomes strongly immobilized after the polymerization of tubulin. The amino acids that interact with the α- and β- tubulin are slightly different depending on the assembled isotypes. The amino acids that make up the <scene name='80/809872/Sami_scene_8/3'>binding site on β-tubulin</scene> include Asp226, His229, Val23, Arg369, Gly370, Thr276 and Arg278. The strong interaction allows for the binding of <scene name='80/809872/Sami_scene_9/6'>Paclitaxel to β tubulin</scene>. The amino acids involved with the binding of Paclitaxel to the <scene name='80/809872/Sami_scene_10/4'>extended protein loop in α- tubulin</scene> include Arg229, Leu23, Leu26, Arg320, Pro360, Tyr272, Lys370, Ala369, Ala278 and Leu217. The strong interaction allows for the binding of <scene name='80/809872/Sami_scene_11/3'>Paclitaxel To α Tubulin</scene>. The ‘stickiness’ of the M loop side of the protofilament appears to be largely responsible for the polymorphic nature of the protofilaments. Whilst the microtubules remain assembled, an observed 3-6% shortening of the ~4nm average spacing of tubulin monomers, is thought to be responsible for stabilizing the microtubule polymer and protecting it from disassembly. Chromosomes are thus unable to achieve a metaphase spindle configuration. <ref name="linda" /> |
The <scene name='80/809872/Sami_scene_1/16'>polarity of α and β tubulin upon the binding of Paclitaxel</scene> is colored according to whether it is polar or nonpolar; pink representing polar, grey representing nonpolar. Before Paclitaxel binds, the binding pocket is highly nonpolar (all grey in color). Once the binding center is occupied by Paclitaxel, it shows excellent shape complementarity. The addition of some pink in the diagram illustrates that a nonpolar depression has been converted to a polar surface upon Paclitaxel binding. <ref>DOI: 10.1073/pnas.051309398</ref> | The <scene name='80/809872/Sami_scene_1/16'>polarity of α and β tubulin upon the binding of Paclitaxel</scene> is colored according to whether it is polar or nonpolar; pink representing polar, grey representing nonpolar. Before Paclitaxel binds, the binding pocket is highly nonpolar (all grey in color). Once the binding center is occupied by Paclitaxel, it shows excellent shape complementarity. The addition of some pink in the diagram illustrates that a nonpolar depression has been converted to a polar surface upon Paclitaxel binding. <ref>DOI: 10.1073/pnas.051309398</ref> | ||
| Line 40: | Line 38: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | *[[:Category:Anti-cancer|Anti-Cancer]] | ||
| + | *[[:Category:Cancer|Cancer]] | ||
| + | *[[:Category:Cancer therapeutic|Cancer Therapeutic]] | ||
Current revision
The Interaction of Paclitaxel with Microtubules
| |||||||||||