We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Uncoupling Protein 2
From Proteopedia
(Difference between revisions)
m (Kimberly Jimenez: Uncoupling Protein 2 moved to Uncoupling Protein 2: Author agreed to the formal new name) |
|||
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Uncoupling Protein 2 (UCP2) in Diabetes== | ==Uncoupling Protein 2 (UCP2) in Diabetes== | ||
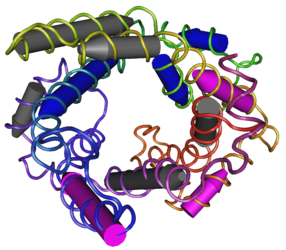
| - | <StructureSection load='2LCK' size='500' side='right' caption=' | + | <StructureSection load='2LCK' size='500' side='right' caption='NMR structure of mouse uncoupling protein 2 (PDB code [[2lck]])' scene=''> |
| - | + | ||
| - | + | ||
| - | [[Image:286px-MMDB_ID_92271_PDB_ID_2LCK_Mitochondrial_Uncoupling_Protein_2.png]] | ||
| - | + | == Introduction == | |
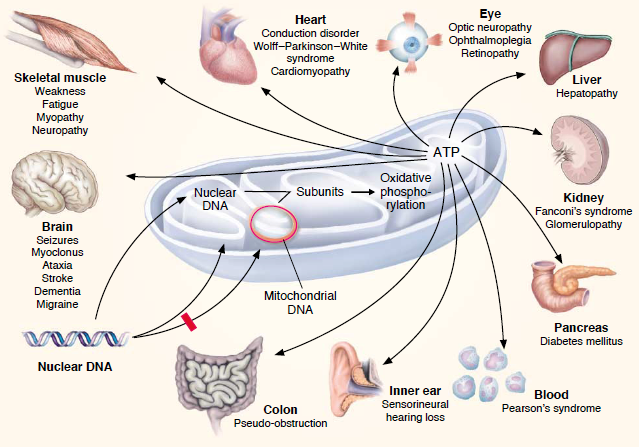
| + | Mutations involving the mitochondria can lead to varies diseases. One particular disease that involves a 3243G mutation in the mitochondrial DNA-encoded tRNA gene is known as Diabetes Mellitus (DM). The predominant issues seen in patients with this disease is glucose intolerances due to gradual development of pancreatic beta-cell malfunction which can be seen with increased age. '''Uncoupling proteins''' are key factors that generate these issues. Low levels of uncoupling proteins (UCPs) can lead to high mitochondrial membrane potentials. These increased potentials favor generation of reactive oxygen species (ROS). Increased ROS levels assist the progress of apoptosis and the differentiation state of pancreatic beta-cells which ultimately lead to inadequate secretion of insulin and an inability to maintain glucose levels. Five UCPs have been discovered in mammals, but UCP1, UCP2 and UCP3 have the biggest roles in DM. UPC1’s underlying determinant of DM is from an imbalance of energy intake and expenditure. Research has also found that polymorphisms and UCP1’s mRNA expressions are highly associated with DM. UCP2, the main focus of this research, is known as a negative regulator of insulin secretion and plays the biggest role in DM generation. The ROS initiate UCP2 which is preceded by decreased beta-cell ATP synthesis and lack of regulation of glucose-stimulating insulin secretion. Polymorphism of UCP2 gene is also correlated with DM. UCP3 is also shown to influence insulin secretion through these beta-cells, but less research has been conducted on these proteins. | ||
| + | |||
| + | == History == | ||
| + | In 1963, researchers discovered that mitochondria have their own DNA or "blueprint" (mtDNA), which is different than the nuclear DNA (nDNA) found in the cells' nucleus. <ref>Mitochondrial Disorder Medical Information. http://www.mitoaction.org/medical-information.</ref> Later, in 1988, mutations of this mitochondria DNA was discovered. Mutations like deletions and point mutations were found in all the mitochondrial disorders.<ref>Luft, R.; Luthman, H. Physiopathology of mitochondria. From Luft's disease to aging and diabetes. https://www.ncbi.nlm.nih.gov/pubmed/8366714.</ref> The discovery of five UCPs are of great importance to the study of the mitochondria. A sequence relating to UCP1 lead to the ultimate discovery of UCP2 in 1997. <ref>Sreedhar, A.; Zhao, Y. Uncoupling protein 2 and metabolic diseases. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5477468/</ref> | ||
== Disease == | == Disease == | ||
| Line 19: | Line 20: | ||
| - | The disorder which affects the pancreas is caused by the A3243G mutation which causes diabetes. | + | The disorder which affects the pancreas is caused by the A3243G mutation which causes diabetes. Bilateral hearing impairment is a symptom carried by the carrier of mitochondrial diabetes. Some other symptoms that can be seen is a change in pigmentation of the retina. <ref>Maassen, J. A.; Leen; Hart; Essen, E. van; Heine, R. J.; Nijpels, G.; Tafrechi, R. S. J.; Raap, A. K.; Janssen, G. M. C.; Lemkes, H. H. P. J. Mitochondrial Diabetes. http://diabetes.diabetesjournals.org/content/53/suppl_1/S103.</ref> This mutation can be seen around the age of 38 years old, this disease is age dependent in the fact that over time there is a deterioration of glucose homeostasis. The pattern of inhertitance of this disorder depends on the mitochondria of the mother and can be passed down to both male and female offspring. |
| + | |||
== Function == | == Function == | ||
| + | Uncoupling Protein 2 (UCP2), as seen in figure 2, is found throughout the body in many tissues, mostly being expressed in the spleen, lung, kidney and in insulin-producing pancreatic islet β cells. While it’s exact function is still unclear, there is increasing amounts of evidence which suggests its main functions include mitigation of reactive oxygen species (ROS) generation and regulation of insulin release.<ref>Sivitz, W. I.; Yorek, M. A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2824521/.</ref> The mitochondria produce large portions of the cell’s overall ROS. UCP2’s function is to bring upon a defense mechanism which diminishes mitochondrial ROS formation by the complexes I and III. As for insulin secretion, UCP2 is considered a negative regulator for insulin secretion.<ref>Chan, C. B.; Saleh, M. C.; Koshkin, V.; Wheeler, M. B. Uncoupling Protein 2 and Islet Function. http://diabetes.diabetesjournals.org/content/53/suppl_1/S136.</ref> This is because ATP generation is essential for the process of opening potassium ATP channels which allow calcium and insulin to be released from storage granules. UCP2 acts to deplete ATP productions which ultimately effects these ATP/ADP ratios that controls insulin secretion. When UCP2 is inactivated, the ATP/ADP ratio increases leading to the activation of glucose-induced insulin secretion. When UCP2 is overexpressed, insulin secretion is decreased.<ref>Sophie Rousset, M.-C. A.-G.; Mozo, J.; Bruno Miroux, A.-M. C.-D.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. http://diabetes.diabetesjournals.org/content/53/suppl_1/S130.</ref> | ||
| + | |||
| + | |||
| + | [[Image:286px-MMDB_ID_92271_PDB_ID_2LCK_Mitochondrial_Uncoupling_Protein_2.png]] | ||
| + | Figure 2: Uncoupling Protein 2 | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Uncoupling Protein 2 (UCP2) in Diabetes
| |||||||||||
References
- ↑ Mitochondrial Disorder Medical Information. http://www.mitoaction.org/medical-information.
- ↑ Luft, R.; Luthman, H. Physiopathology of mitochondria. From Luft's disease to aging and diabetes. https://www.ncbi.nlm.nih.gov/pubmed/8366714.
- ↑ Sreedhar, A.; Zhao, Y. Uncoupling protein 2 and metabolic diseases. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5477468/
- ↑ Maassen, J. A.; Leen; Hart; Essen, E. van; Heine, R. J.; Nijpels, G.; Tafrechi, R. S. J.; Raap, A. K.; Janssen, G. M. C.; Lemkes, H. H. P. J. Mitochondrial Diabetes. http://diabetes.diabetesjournals.org/content/53/suppl_1/S103.
- ↑ Sivitz, W. I.; Yorek, M. A. Mitochondrial dysfunction in diabetes: from molecular mechanisms to functional significance and therapeutic opportunities. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2824521/.
- ↑ Chan, C. B.; Saleh, M. C.; Koshkin, V.; Wheeler, M. B. Uncoupling Protein 2 and Islet Function. http://diabetes.diabetesjournals.org/content/53/suppl_1/S136.
- ↑ Sophie Rousset, M.-C. A.-G.; Mozo, J.; Bruno Miroux, A.-M. C.-D.; Bouillaud, F.; Ricquier, D. The Biology of Mitochondrial Uncoupling Proteins. http://diabetes.diabetesjournals.org/content/53/suppl_1/S130.