User:Emily Leiderman/Sandbox 1
From Proteopedia
< User:Emily Leiderman(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 22: | Line 22: | ||
=== Hydrophobic Pocket === | === Hydrophobic Pocket === | ||
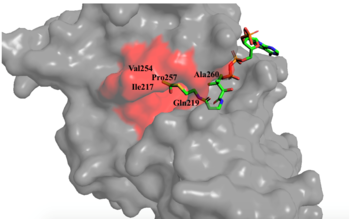
[[Image:hydroophobic pocket.png|350px|right|thumb|Figure 1. Image of the substrate Acetyl CoA bound in the groove of HAT1 (1BOB.pdb). The groove contains the three residues involved in the acetyltransferase mechanism.]] The active site consists of the Acetyl CoA ligand bound to the enzyme in a groove on the surface of the protein. The [https://en.wikipedia.org/wiki/Ligand ligand] is held in place by several bonds to protein residues that result in the formation of a <scene name='81/811098/Hydrophobic_pocket/2'>hydrophobic pocket</scene>. The hydrophobic pocket consists of the interacting side chains from residues <scene name='81/811098/Ile-217_pro-257_phe-261/3'>Ile-217, Pro-257, and Phe-261</scene> in addition to further bonds resulting from residues 217-220 and 255-256 <ref name=Dut/> (figure 1). The amide of main-chain <scene name='81/811098/Phe-220/4'>Phe-220</scene> hydrogen bonds to the carbonyl oxygen of the Acetyl group in the binding pocket. The main-chain amide of <scene name='81/811098/Asn-258/4'>Asn-258</scene> also donates a hydrogen bond from its side chain to oxygen PO5 of the pantothenic acid group <ref name=Dut/>. The binding within the hydrophobic pocket is further supplemented through the creation of a protein gate that establishes a bridge over the concave surface that serves to keep Acetyl CoA in place while the enzyme interacts with the histone. | [[Image:hydroophobic pocket.png|350px|right|thumb|Figure 1. Image of the substrate Acetyl CoA bound in the groove of HAT1 (1BOB.pdb). The groove contains the three residues involved in the acetyltransferase mechanism.]] The active site consists of the Acetyl CoA ligand bound to the enzyme in a groove on the surface of the protein. The [https://en.wikipedia.org/wiki/Ligand ligand] is held in place by several bonds to protein residues that result in the formation of a <scene name='81/811098/Hydrophobic_pocket/2'>hydrophobic pocket</scene>. The hydrophobic pocket consists of the interacting side chains from residues <scene name='81/811098/Ile-217_pro-257_phe-261/3'>Ile-217, Pro-257, and Phe-261</scene> in addition to further bonds resulting from residues 217-220 and 255-256 <ref name=Dut/> (figure 1). The amide of main-chain <scene name='81/811098/Phe-220/4'>Phe-220</scene> hydrogen bonds to the carbonyl oxygen of the Acetyl group in the binding pocket. The main-chain amide of <scene name='81/811098/Asn-258/4'>Asn-258</scene> also donates a hydrogen bond from its side chain to oxygen PO5 of the pantothenic acid group <ref name=Dut/>. The binding within the hydrophobic pocket is further supplemented through the creation of a protein gate that establishes a bridge over the concave surface that serves to keep Acetyl CoA in place while the enzyme interacts with the histone. | ||
| + | |||
| + | |||
| Line 36: | Line 38: | ||
=== Protein Gate === | === Protein Gate === | ||
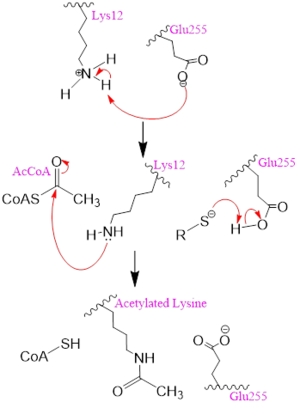
| - | The <scene name='81/811098/Gate/5'>protein gate</scene> consists of several residues that coordinate a conformational change in the protein to hold the Acetyl CoA ligand in place. <scene name='81/811098/161_162_258/1'> | + | The <scene name='81/811098/Gate/5'>protein gate</scene> consists of several residues that coordinate a conformational change in the protein to hold the Acetyl CoA ligand in place. <scene name='81/811098/161_162_258/1'>Asn-258, Glu-162, Ile-161</scene> cooperate to create a bridge over Acetyl CoA <ref name=Dut/>. The main chain amide of <scene name='81/811098/Phe-261_asn-258/1'>Phe-261</scene> hydrogen bonds to the carbonyl oxygen of Asn-258 which allows for proper positioning of Asn-258 to bond with other residues and Acetyl CoA itself. The side chain amide of Asn-258 also binds via a water molecule (H2O-415) to Glu-162 on its main chain amide <ref name=Dut/>. These bonds help secure the position of <scene name='81/811098/Asn-258/4'>Asn-258</scene> so that a hydrogen bond can be established from the side-chain amide to the PO5 carbonyl oxygen. Together, the side chains of Ile-161, Glu-162, and Asn-258 form a protein gate over the Acetyl CoA binding cleft. Hydrogen bonds further fixate Acetyl CoA by connecting the main chain amide of Glu-162 to the side chain amide of Asn-258. The side chain carbonyl oxygen of Phe-261 hydrogen bonds to its main chain amide to bridge the binding cleft over Acetyl-CoA. The gate allows for correct binding of Acetyl CoA. Once the ligand is bound, subsequent conformational changes of HAT1 take place allowing for Lys-12 to act as a nucleophile. |
| + | |||
== Medical Relevance == | == Medical Relevance == | ||
| + | Histone acetyltransferases consists of 5 families: GNAT, MYST, p300/CBP, and Rtt109. HATs of these families have histone substrate specificity, such as lysine, and non-histone substrate specificity, such as proteins and transcription factors. Specifically, the acetylation of the particular residue, lysine 16 of histone H4, is crucial in maintaining a normal [https://en.wikipedia.org/wiki/LMNA lamin A] gene and genomic stability since a mutation in the lamin A gene can cause premature, rapid aging <ref name=idkk>PMID:21746928 </ref>. [https://en.wikipedia.org/wiki/progeria Hutchinson Gilford progeria], an example of an accelerated aging syndrome, is caused by a mutated lamin A producing the mutant protein [https://en.wikipedia.org/wiki/Progerin progerin], resulting in the delayed response time of [https://en.wikipedia.org/wiki/DNA_repair repair proteins] during DNA damage <ref name=idkk/>. A study done by Krishnan et. al showed that it was a defect in histone acetyltransferase, Mof, in [https://en.wikipedia.org/wiki/ZMPSTE24 Zmpste24-/-] mice that allowed for the hypoacetylation of lysine 16 residue (H4K16) which lead to a delayed response in modified histones recruiting repair proteins to fix DNA damage. Thus, the inability of repair proteins to correct DNA damage suggests that cells get inclined to rapid aging and early [https://en.wikipedia.org/wiki/Senescence senescence], resulting in syndromes such as Hutchinson Gilford progeria <ref name=idkk/>. | ||
Current revision
Histone Acetyltransferase 1
| |||||||||||