We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Madeleine Wilson/Sandbox 1
From Proteopedia
< User:Madeleine Wilson(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 20: | Line 20: | ||
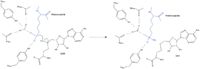
The active site and binding pocket of KMT contain residues and shape that optimize catalytic function and stability. First, the lysine of the histone enters the active site via the <scene name='81/811092/Tyrosine_channel_2/3'>Lysine access channel</scene> comprised of Tyr335 and Tyr337. Feeding the histone into the active site is initially difficult; however, once in the active site, the alkyl part of the histone chain is stabilized by the <scene name='81/811092/Hydrophobic_packing/4'>hydrophobic binding pocket</scene>, and polar residues are stabilized by hydrogen bonding interactions on the surface. The Tyr335 and Tyr337 are also essential for stabilization of histone chain via hydrogen bonding. The <scene name='81/811092/Active_site_w_water/3'>active site</scene> itself contains the cofactor [https://en.wikipedia.org/wiki/S-Adenosyl_methionine S-adenosyl methionine (SAM)] which donates the methyl group in the reaction. <ref name="Xiao" /> In the active site scene, the structures depict the post-reaction result, where the Lys has been methylated and SAM has been converted to S-adenosyl homocysteine (SAH). | The active site and binding pocket of KMT contain residues and shape that optimize catalytic function and stability. First, the lysine of the histone enters the active site via the <scene name='81/811092/Tyrosine_channel_2/3'>Lysine access channel</scene> comprised of Tyr335 and Tyr337. Feeding the histone into the active site is initially difficult; however, once in the active site, the alkyl part of the histone chain is stabilized by the <scene name='81/811092/Hydrophobic_packing/4'>hydrophobic binding pocket</scene>, and polar residues are stabilized by hydrogen bonding interactions on the surface. The Tyr335 and Tyr337 are also essential for stabilization of histone chain via hydrogen bonding. The <scene name='81/811092/Active_site_w_water/3'>active site</scene> itself contains the cofactor [https://en.wikipedia.org/wiki/S-Adenosyl_methionine S-adenosyl methionine (SAM)] which donates the methyl group in the reaction. <ref name="Xiao" /> In the active site scene, the structures depict the post-reaction result, where the Lys has been methylated and SAM has been converted to S-adenosyl homocysteine (SAH). | ||
[[Image:KMT_Mechanism_jpg.jpeg|200px|left|thumb|Figure 2. KMT Mechanism]] | [[Image:KMT_Mechanism_jpg.jpeg|200px|left|thumb|Figure 2. KMT Mechanism]] | ||
| - | The reaction is catalyzed by Tyr305, Tyr245, carbonyl oxygens of the main chain in residues Ala295 and Ser290. Tyr305 and the carbonyl oxygens of Ala295 and Set 290 coordinate with a water molecule to in turn coordinate with one of the hydrogens off the nitrogen of the lysine, while oxygen of Tyr245 pulls on the other hydrogen of the nitrogen. Both of these actions allow nitrogen to become more nucleophilic and attack the carbon of the methyl group on the SAM, which is attached to a positively charged sulfur. The methyl group is then transferred and charge on the sulfur is | + | The reaction is catalyzed by Tyr305, Tyr245, carbonyl oxygens of the main chain in residues Ala295 and Ser290. Tyr305 and the carbonyl oxygens of Ala295 and Set 290 coordinate with a water molecule to in turn coordinate with one of the hydrogens off the nitrogen of the lysine, while oxygen of Tyr245 pulls on the other hydrogen of the nitrogen. Both of these actions allow nitrogen to become more nucleophilic and attack the carbon of the methyl group on the SAM, which is attached to a positively charged sulfur. The methyl group is then transferred and charge on the sulfur is resolved; SAM has been converted to SAH. <ref name="Xiao" /> |
===The C-Terminal Domain=== | ===The C-Terminal Domain=== | ||
| - | The C-terminal domain of lysine methyltransferase is very important for the catalytic activity of the enzyme. The structures of the <scene name='81/811091/C_terminal_domain/1'>C-terminal domain (residues 345-366)</scene> serve the role of stabilizing the structures in the <scene name='81/811091/C_terminal_domain/16'>SET7 domain (residues 193-344)</scene> in the correct orientation for a reaction in the active site.<ref name="Xiao" /> Hydrophobic interactions in the C-terminal domain are mainly responsible for stabilizing the access channel for the lysine methylation site on histone H3. Residues 337-349 create a <scene name='81/811086/Beta-hairpin/1'>beta-hairpin</scene> that stabilizes the orientation of two tyrosine residues Tyr 335 and Tyr337 that form the lysine access channel. Furthermore, the hydrophobic packing of alpha-helix 3 against beta-sheet 19, specifically <scene name='81/811091/C_terminal_domain/13'>residues Leu357 and Phe 299</scene>, stabilize the orientation of the <scene name='81/811091/C_terminal_domain/ | + | The C-terminal domain of lysine methyltransferase is very important for the catalytic activity of the enzyme. The structures of the <scene name='81/811091/C_terminal_domain/1'>C-terminal domain (residues 345-366)</scene> serve the role of stabilizing the structures in the <scene name='81/811091/C_terminal_domain/16'>SET7 domain (residues 193-344)</scene> in the correct orientation for a reaction in the active site.<ref name="Xiao" /> Hydrophobic interactions in the C-terminal domain are mainly responsible for stabilizing the access channel for the lysine methylation site on histone H3. Residues 337-349 create a <scene name='81/811086/Beta-hairpin/1'>beta-hairpin</scene> that stabilizes the orientation of two tyrosine residues Tyr 335 and Tyr337 that form the lysine access channel. Furthermore, the hydrophobic packing of alpha-helix 3 against beta-sheet 19, specifically <scene name='81/811091/C_terminal_domain/13'>residues Leu357 and Phe 299</scene>, stabilize the orientation of the <scene name='81/811091/C_terminal_domain/20'>SAM cofactor</scene> so that its methyl donating group is oriented toward the lysine access channel. The orientation of the SAM cofactor is further stabilized with its hydrophobic interactions with C-terminal domain residues <scene name='81/811092/C_terminal_domain/1'>Glu356 and Trp352</scene>. <ref name="Xiao" /> |
===The N-terminal Domain=== | ===The N-terminal Domain=== | ||
| Line 31: | Line 31: | ||
==Inhibitors== | ==Inhibitors== | ||
| - | + | ||
| - | Sinefungin is a potent methyltransferase inhibitor. | + | SET7/9’s structure and function has been studied extensively because of its role in transcription <ref name="Takemoto">PMID:27088648</ref>. In the past few years it has been identified to methylate genes involved in multiple diseases; making it a potential candidate for drug inhibition. <ref name="Tamura">PMID:29723250</ref> Two compounds that have been found to inhibit SET7/9 in certain cells in vitro are Sinefungin and Cyproheptadine. Each inhibitor acts on the catalytic center of SET7/9, however their mechanisms of inhibition and possible medical relevancies differ greatly. |
| + | |||
| + | ===Sinefungin=== | ||
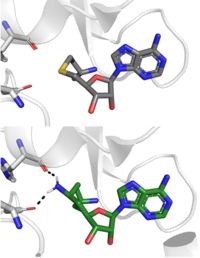
| + | Sinefungin is a potent methyltransferase inhibitor that is a natural nucleoside isolated from the [https://www.britannica.com/science/Streptomyces "Streptomyces"] species. <ref name="Tamura" /> Also referred to as adenosyl-ornithine, it is the delta (5’ adenosyl) derivative of [https://en.wikipedia.org/wiki/Ornithine ornithine] and a [https://en.wikipedia.org/wiki/Structural_analog structural analog] of S-adenosylmethionine. Sinefugin is unique because it inhibits where the cofactor binds rather than where the substrate binds like a typical competitive inhibitor. Sinefungin is more stable bound in the active site than SAH due to the ability to create two additional hydrogen bonds to its amine group that are not possible with SAH’s sulfur. | ||
| + | |||
| + | [[Image: SinSAH.jpg|200 px| right| thumb|SAH (grey) and Sinefungin (green) in the peptide binding pocket. The nitrogen group of sinefungin makes 2 double bonds to the main chain carbonyls of Arg265 and His293. Sinefungin was created using PDB: 1O9S and mutating the sulfur of SAH]] | ||
| + | |||
| + | Sinefungin has been used experimentally to inhibit the SET 7/9 protein on [https://www.sciencedirect.com/topics/medicine-and-dentistry/peritoneal-fibrosis peritoneal fibrosis] in mice and in human peritoneal mesothelial cells. <ref name="Tamura" /> SET 7/9 is involved in peritoneal fibrosis because it mono-methylates [https://epigenie.com/key-epigenetic-players/histone-proteins-and-modifications/histone-h3k4/ H3K4], which activates the transcription of fibrosis related genes. The administration of Sinefungin to mice in vitro resulted in decreased levels of methylated H3K4 (H3K4me1) protein, as well as suppressed peritoneal cell density and thickening. The decreased levels of H3K4me1 suggest that the methylation of H3K4 was inhibited by Sinefungin, as well as that inhibiting SET7/9 ameliorates peritoneal fibrosis. | ||
| + | |||
| + | ===Cyproheptadine=== | ||
| + | Another inhibitor of SET 7/9 is <scene name='81/811086/Cyproheptadine/3'>cyproheptadine</scene>, a clinically-approved anti-allergy drug that was originally developed as a serotonin and histimine. <ref name="Takemoto" /> The cyproheptadine-SET 7/9 complex was crystallized via X-ray diffraction at 2.005 Å with methylated cofactor SAM and with cydroheptadine. Unlike Sinefungin, it is a traditional competitor and competitive with the peptide substrates as it binds to the peptide-binding site. When cyproheptadine binds to the substrate site, the nitrogen of the [https://www.koeichem.com/en/product/index.php/item?cell003=Amines&cell004=Piperidine+derivatives&page=8&name=N-Methylpiperidine&id=116&label=1 methylpiperdine] ring of cyproheptadine forms a hydrogen bond with Thr286 as well as hydrophobic and interactions with the residues surrounding its binding site. The binding of cyproheptadine to the catalytic site causes conformational changes of residue Tyr337, an important residue for the formation of the lysine access channel. This movement subsequently causes a conformational change of the <scene name='81/811086/Betahairincypr/1'>beta hairpin</scene>. The <scene name='81/811086/Betahairpincyp/3'>residues 337-349</scene> conformational change ultimately generates a large hole adjacent to the lysine access channel, as well as the shift of the C-terminal helix.<ref name="Takemoto" /> | ||
| + | With the revelation of its inhibitory effects on SET7/9, cyproheptadine was used in vitro to treat breast cancer cells ([https://en.wikipedia.org/wiki/MCF-7 MCF-7] cells). SET 7/9's non-histone activities include the methylation of the estrogen receptor α (ERα), a nuclear receptor and a transcription factor responsible for estrogen-responsive gene regulation. The expression and transcriptional activity of ERα is involved in the carcinogenesis of 70% of breast cancers, making it a major target for hormone therapy. Researchers found that treating the MCF7 cells with cyproheptadine decreased ERα's expression and transcriptional activity which therefore inhibited the estrogen-dependent cell growth. These findings suggest that cyproheptadine could possibly be repurposed to breast cancer therapy in the future.<ref name="Takemoto" /> | ||
| + | |||
</StructureSection> | </StructureSection> | ||
Current revision
Histone Lysine Methyltransferase: Gene Activator
| |||||||||||
References
- ↑ DesJarlais R, Tummino PJ. Role of Histone-Modifying Enzymes and Their Complexes in Regulation of Chromatin Biology. Biochemistry. 2016 Mar 22;55(11):1584-99. doi: 10.1021/acs.biochem.5b01210. Epub , 2016 Jan 26. PMID:26745824 doi:http://dx.doi.org/10.1021/acs.biochem.5b01210
- ↑ 2.0 2.1 doi: https://dx.doi.org/10.1016/j.apsb.2013.04.007
- ↑ 3.0 3.1 Dong X, Weng Z. The correlation between histone modifications and gene expression. Epigenomics. 2013 Apr;5(2):113-6. doi: 10.2217/epi.13.13. PMID:23566087 doi:http://dx.doi.org/10.2217/epi.13.13
- ↑ 4.0 4.1 Del Rizzo PA, Trievel RC. Substrate and product specificities of SET domain methyltransferases. Epigenetics. 2011 Sep 1;6(9):1059-67. doi: 10.4161/epi.6.9.16069. Epub 2011 Sep, 1. PMID:21847010 doi:http://dx.doi.org/10.4161/epi.6.9.16069
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 Xiao B, Jing C, Wilson JR, Walker PA, Vasisht N, Kelly G, Howell S, Taylor IA, Blackburn GM, Gamblin SJ. Structure and catalytic mechanism of the human histone methyltransferase SET7/9. Nature. 2003 Feb 6;421(6923):652-6. Epub 2003 Jan 22. PMID:12540855 doi:10.1038/nature01378
- ↑ 6.0 6.1 Kwon T, Chang JH, Kwak E, Lee CW, Joachimiak A, Kim YC, Lee J, Cho Y. Mechanism of histone lysine methyl transfer revealed by the structure of SET7/9-AdoMet. EMBO J. 2003 Jan 15;22(2):292-303. PMID:12514135 doi:http://dx.doi.org/10.1093/emboj/cdg025
- ↑ 7.0 7.1 7.2 7.3 Takemoto Y, Ito A, Niwa H, Okamura M, Fujiwara T, Hirano T, Handa N, Umehara T, Sonoda T, Ogawa K, Tariq M, Nishino N, Dan S, Kagechika H, Yamori T, Yokoyama S, Yoshida M. Identification of Cyproheptadine as an Inhibitor of SET Domain Containing Lysine Methyltransferase 7/9 (Set7/9) That Regulates Estrogen-Dependent Transcription. J Med Chem. 2016 Apr 28;59(8):3650-60. doi: 10.1021/acs.jmedchem.5b01732. Epub, 2016 Apr 18. PMID:27088648 doi:http://dx.doi.org/10.1021/acs.jmedchem.5b01732
- ↑ 8.0 8.1 8.2 Tamura R, Doi S, Nakashima A, Sasaki K, Maeda K, Ueno T, Masaki T. Inhibition of the H3K4 methyltransferase SET7/9 ameliorates peritoneal fibrosis. PLoS One. 2018 May 3;13(5):e0196844. doi: 10.1371/journal.pone.0196844., eCollection 2018. PMID:29723250 doi:http://dx.doi.org/10.1371/journal.pone.0196844
Student Contributors
Lauryn Padgett, Alexandra Pentala, Madeleine Wilson