User:Caitlin Marie Gaich/Sandbox1
From Proteopedia
< User:Caitlin Marie Gaich(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 14: | Line 14: | ||
= Hat1/Hat2 Complex Structure = | = Hat1/Hat2 Complex Structure = | ||
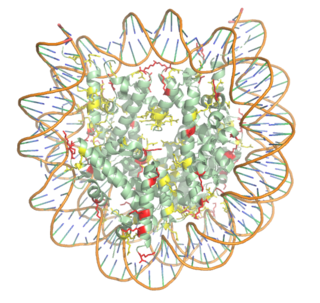
| - | The HAT1/HAT2 complex was | + | The HAT1/HAT2 complex was determine through crystallization and X-ray diffraction using 2.0 Angstrom resolution. The complex was crystallized in the presence of coenzyme A. The complex has four components (HAT1, HAT2, H4, and CoA) seen with a 1:1:1:1 stoichiometry. While residues 1-48 of the yeast H4 were cystallized, only residues 7-46 were well defined. In HAT1, residues 1-7 and residues 319-320 could not be located. Similarly, in HAT2, residues 1-8, residues 86-105, and residues 387-401 were not seen <ref name="Yang"/>. |
| - | + | HAT1 is not catalytically active until it binds with HAT2 to form the <scene name='81/811717/Complex/7'>complex</scene> <ref name="Wu"> PMID: 22615379 </ref>. HAT1 structure, identified as <scene name='81/811717/Hat1_-_chain_a/2'>chain A</scene>, includes 317 residues and contains the binding site for acetyl-coenzyme A. HAT2 is identified as <scene name='81/811717/Hat2_-_chain_b/3'>chain B</scene>, which includes 401 residues in a beta-propeller formation with C7 symmetry. Bound to this complex is the histone protein <scene name='81/811717/Histone_4/3'>H4</scene> residues 1-38. | |
The HAT1 and HAT2 interface is stabilized by several interactions of multiple types. Most of these interactions are located in a HAT1 <scene name='81/811717/Lp1/5'>short, well-ordered helix</scene> of residues 200-208. This helix is thought to be important for the heterodimer formation as the deletion of the helix abolishes the interaction between HAT1 and HAT2. This suggests that there may be another protein involved, such as the N terminus tail of H4, acting as a linker protein interacting with the complex interface to further stabilize the complex interface <ref name="Yang" />. This HAT1/HAT2 interface is stabilized by <scene name='81/811717/Salt_bridges/4'>salt bridges</scene> between the two subunits. There are three major areas where hydrogen bonds are present aids in this complex formation. The side chain atoms of <scene name='81/811717/Tyr199_asp308_ala202/7'>Tyr199 and Asp308</scene> with the main chain nitrogen of Ala202 in HAT1. The side chain of <scene name='81/811717/Lys211phe205_and_leu288arg282/9'>Lys211 and Arg282</scene> makes hydrogen bonds with Leu288 and Phe205 respectively. The last area of hydrogen bonds between HAT1 and HAT is found between <scene name='81/811717/Serine_hydrogen_bonds/4'>Ser263 and Asp 206</scene>. The <scene name='81/811717/Hydrophobic_core/4'>hydrophobic core</scene> at the interface of the complex appears to be critical for the complex formation. This core consists of aromatic amino acids from HAT1 and leucine amino acids from HAT2, however it does not form any obvious ring stacking. | The HAT1 and HAT2 interface is stabilized by several interactions of multiple types. Most of these interactions are located in a HAT1 <scene name='81/811717/Lp1/5'>short, well-ordered helix</scene> of residues 200-208. This helix is thought to be important for the heterodimer formation as the deletion of the helix abolishes the interaction between HAT1 and HAT2. This suggests that there may be another protein involved, such as the N terminus tail of H4, acting as a linker protein interacting with the complex interface to further stabilize the complex interface <ref name="Yang" />. This HAT1/HAT2 interface is stabilized by <scene name='81/811717/Salt_bridges/4'>salt bridges</scene> between the two subunits. There are three major areas where hydrogen bonds are present aids in this complex formation. The side chain atoms of <scene name='81/811717/Tyr199_asp308_ala202/7'>Tyr199 and Asp308</scene> with the main chain nitrogen of Ala202 in HAT1. The side chain of <scene name='81/811717/Lys211phe205_and_leu288arg282/9'>Lys211 and Arg282</scene> makes hydrogen bonds with Leu288 and Phe205 respectively. The last area of hydrogen bonds between HAT1 and HAT is found between <scene name='81/811717/Serine_hydrogen_bonds/4'>Ser263 and Asp 206</scene>. The <scene name='81/811717/Hydrophobic_core/4'>hydrophobic core</scene> at the interface of the complex appears to be critical for the complex formation. This core consists of aromatic amino acids from HAT1 and leucine amino acids from HAT2, however it does not form any obvious ring stacking. | ||
| Line 35: | Line 35: | ||
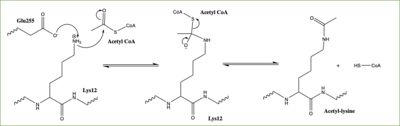
[[Image:HAT1_Mechanism.jpg|400px|right|thumb|Figure 3: Proposed HAT1 Mechanism. E255 acts as a general base to deprotonate K12 of H4]] | [[Image:HAT1_Mechanism.jpg|400px|right|thumb|Figure 3: Proposed HAT1 Mechanism. E255 acts as a general base to deprotonate K12 of H4]] | ||
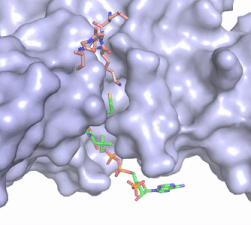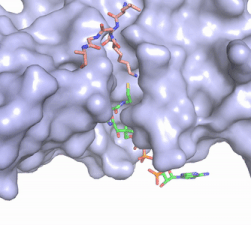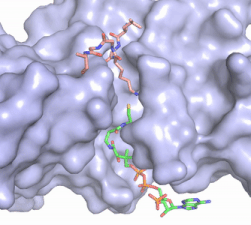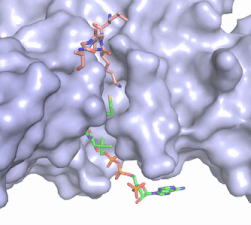
| - | In this mechanism, the glutamate at residue 255, a general base in this mechanism, in the active site of the protein acts to deprotonate lysine 12 of histone 4 (the numbering of the modified lysine residue on histone 4 is shifted two residues in the featured structure).Acting as the nucleophile, the | + | In this mechanism, the glutamate at residue 255, a general base in this mechanism, in the active site of the protein acts to deprotonate lysine 12 of histone 4 (the numbering of the modified lysine residue on histone 4 is shifted two residues in the featured structure). Surrounding the lysine residue on histone 4 are <scene name='81/811713/Carbonyl_interactions/1'> carbonyl carbons on the main chain of aspartate at residue 256, glutamate at residue 255, and serine at residue 218</scene> which interact with the amino end of the lysine to better orient the lone pair electrons for nucleophillic attack. Acting as the nucleophile, the lone pair on the lysine attacks the carbonyl carbon of acetyl-CoA (acetyl group not shown in the structure), forming a tetrahedral transition state containing an oxyanion. The negative charge on the oxyanion is then shift to down to reform the double bond between the oxygen and carbonyl carbon, breaking the scissle bond between the carbonyl carbon and the sulfur atom of acetyl CoA. The resulting product of this reaction is histone 4 with an acetyl-lysine at residue 12 and coenzyme A. |
= Inhibition = | = Inhibition = | ||
Current revision
Histone Acetyltransferase HAT1/HAT2 Complex, Saccharomyces cerevisiae
| |||||||||||