HIV-1 Nucleocapsid Protein (NC)
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='1mfs' size='350' side='right' caption='Nucleocapsid Protein'> ==Overview== Nucleocapsid (NC) has an important role in many steps of the Human Immunodeficiency Virus...) |
|||
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='1mfs' size='350' side='right' caption='Nucleocapsid Protein'> | + | <StructureSection load='1mfs' size='350' side='right' caption='Nucleocapsid Protein complex with Zn+2 ions (PDB code [[1mfs]])'> |
==Overview== | ==Overview== | ||
| - | Nucleocapsid (NC) has an important role in many steps of the Human Immunodeficiency Virus 1 (HIV-1) life cycle. NC has been shown to initiate dimerization of HIV-1 genomic RNA (gRNA) and formation of the viral particle. NC also plays a vital role in initiating reverse transcription and elongation of cDNA. Many of these functions are the result of the RNA binding properties of NC as well as NC’s ability to destabilize regions of base paring. NC is an important HIV-1 chaperone. | + | '''Nucleocapsid''' (NC) has an important role in many steps of the Human Immunodeficiency Virus 1 (HIV-1) life cycle. NC has been shown to initiate dimerization of HIV-1 genomic RNA (gRNA) and formation of the viral particle. NC also plays a vital role in initiating reverse transcription and elongation of cDNA. Many of these functions are the result of the RNA binding properties of NC as well as NC’s ability to destabilize regions of base paring. NC is an important HIV-1 chaperone. |
| + | |||
| + | See also [[Nucleoprotein]]. | ||
== Function == | == Function == | ||
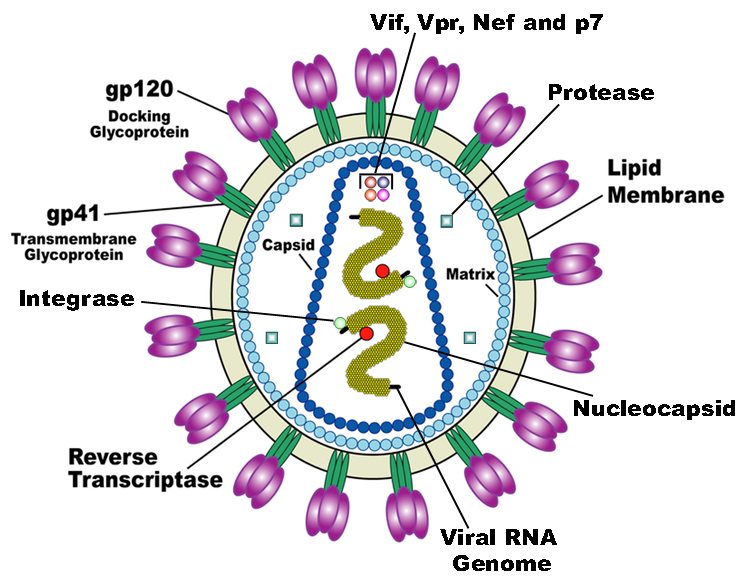
| + | [[Image:HI-Virion-en.png|frame|Structure of HIV-1 Virus Particle <ref>[https://commons.wikimedia.org/wiki/File:HI-Virion-en.png] was obtained from Wikipedia.</ref>.]] | ||
NC facilitates the dimerization of gRNA. HIV-1 genomic information is packaged in the viral particle as a dimer. The palindromic sequence GCGCGC at the dimerization initiation site (DIS) (nt 240-280) initiates complimentary base pairing with another copy of gRNA and forms a kissing loop complex. This kissing loop complex is considered a loose dimer due to its low thermal stability. NC protein facilitates the transition from the low stability kissing loop complex to the stable extended dimer by promoting the refolding of the 5’-end regions via formation of cruciform intermediates (Dubois, Marquet, Paillart, & Bernacchi, 2018). NC makes up the c-terminal end of HIV-1 Gag protein, composed of three domains that is eventually cleaved to form matrix (MA), capsid (CA), and nucleocapsid (NC). Gag is responsible for formation of the final viral particle. | NC facilitates the dimerization of gRNA. HIV-1 genomic information is packaged in the viral particle as a dimer. The palindromic sequence GCGCGC at the dimerization initiation site (DIS) (nt 240-280) initiates complimentary base pairing with another copy of gRNA and forms a kissing loop complex. This kissing loop complex is considered a loose dimer due to its low thermal stability. NC protein facilitates the transition from the low stability kissing loop complex to the stable extended dimer by promoting the refolding of the 5’-end regions via formation of cruciform intermediates (Dubois, Marquet, Paillart, & Bernacchi, 2018). NC makes up the c-terminal end of HIV-1 Gag protein, composed of three domains that is eventually cleaved to form matrix (MA), capsid (CA), and nucleocapsid (NC). Gag is responsible for formation of the final viral particle. | ||
| - | [[Image:HI-Virion-en.png]] | ||
The NC domain of Gag binds to gRNA with high specificity to the <scene name='81/814018/Psi_nc_complex/1'>psi element</scene>. In this way, gRNA serves as a scaffold for the oligomerization of Gag in viral particle formation. NC binding facilitated by the electrostatic interactions between the two zinc fingers and double stranded RNA. NC-gRNA binding is important for gRNA association with and growth of the viral particle as it migrates to the cell plasma membrane (PM). The NC domain initially binds to the gRNA, and then MA domain facilitates Gag migration (along with the bound gRNA) to the cell membrane. This is supported by the fact that NC mutant Gag (Gag with a leucine or isoleucine zipper motif instead of NC, but still able to oligomerize without RNA) localized and oligomerized at the PM but lacked gRNA specificity and would form empty virus like particles (VPLs) (Yang, et al., 2018). Gag proteins lacking the NC domain (∆NC-Gag) has greatly reduced Gag assembly at the PM. It has been shown that Gag binds to gRNA in clusters (dimers and trimers) and then subsequent Gag proteins multimerizes between these Gag clusters (Yang, et al., 2018). NC protein is important for this multimerization as ∆NC-Gag is unable to oligomerize. | The NC domain of Gag binds to gRNA with high specificity to the <scene name='81/814018/Psi_nc_complex/1'>psi element</scene>. In this way, gRNA serves as a scaffold for the oligomerization of Gag in viral particle formation. NC binding facilitated by the electrostatic interactions between the two zinc fingers and double stranded RNA. NC-gRNA binding is important for gRNA association with and growth of the viral particle as it migrates to the cell plasma membrane (PM). The NC domain initially binds to the gRNA, and then MA domain facilitates Gag migration (along with the bound gRNA) to the cell membrane. This is supported by the fact that NC mutant Gag (Gag with a leucine or isoleucine zipper motif instead of NC, but still able to oligomerize without RNA) localized and oligomerized at the PM but lacked gRNA specificity and would form empty virus like particles (VPLs) (Yang, et al., 2018). Gag proteins lacking the NC domain (∆NC-Gag) has greatly reduced Gag assembly at the PM. It has been shown that Gag binds to gRNA in clusters (dimers and trimers) and then subsequent Gag proteins multimerizes between these Gag clusters (Yang, et al., 2018). NC protein is important for this multimerization as ∆NC-Gag is unable to oligomerize. | ||
Current revision
| |||||||||||