This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Cystathionine β-synthase
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==3D Structure of Human Cystathionine β-synthase (4coo)== | ==3D Structure of Human Cystathionine β-synthase (4coo)== | ||
<StructureSection load='4coo' size='340' side='right' caption='Human Cystathionine β-synthase' scene=''> | <StructureSection load='4coo' size='340' side='right' caption='Human Cystathionine β-synthase' scene=''> | ||
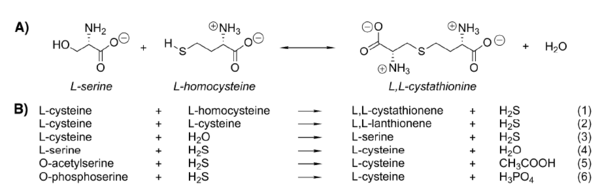
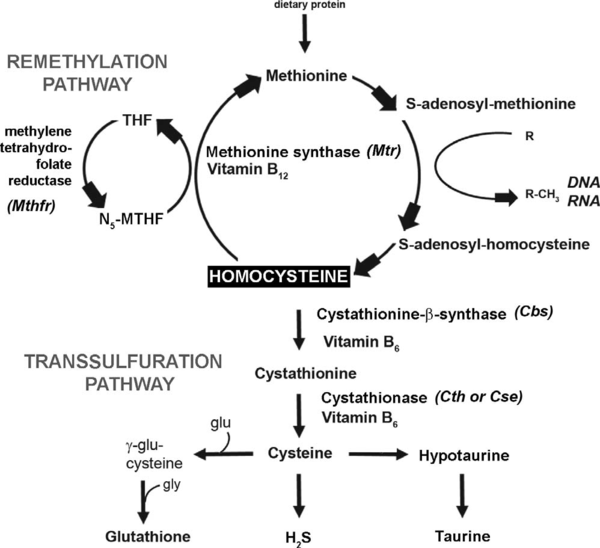
| - | Cystathionine β-synthase (CBS; EC 4.2.1.22) is a unique pyridoxal 5’-phosphate (PLP) enzyme which plays a crucial role in the transsulfuration metabolic pathway of sulfur-containing amino acid, L-homocysteine. | + | '''Cystathionine β-synthase''' (CBS; EC 4.2.1.22) is a unique pyridoxal 5’-phosphate (PLP) enzyme which plays a crucial role in the transsulfuration metabolic pathway of sulfur-containing amino acid, L-homocysteine. |
PLP enzymes are divided into four families on the basis of similarities in three dimensional structure, sequence, secondary structure, and hydrophobicity profiles. CBS is a member of the β family or Fold type II, which also contains O-acetylserine sulfhydrylase (OASS) and O-acetyl-L-serine(thiol)lyase (OASTL)<ref>PMID:11483494</ref>. | PLP enzymes are divided into four families on the basis of similarities in three dimensional structure, sequence, secondary structure, and hydrophobicity profiles. CBS is a member of the β family or Fold type II, which also contains O-acetylserine sulfhydrylase (OASS) and O-acetyl-L-serine(thiol)lyase (OASTL)<ref>PMID:11483494</ref>. | ||
| Line 78: | Line 78: | ||
The first one has centered on the relationship between homocysteine and oxidative stress. Homocysteine itself has been shown to cause increased oxidative stress on cells, both through direct effects (e.g., the production of hydrogen peroxide by oxidation of homocysteine to homocystine) and indirect effects (e.g., reduction of glutathione peroxidase). In addition, it is estimated that as much as 50% of the cellular antioxidant glutathione is produced from homocysteine by conversion through the transsulfuration pathway. | The first one has centered on the relationship between homocysteine and oxidative stress. Homocysteine itself has been shown to cause increased oxidative stress on cells, both through direct effects (e.g., the production of hydrogen peroxide by oxidation of homocysteine to homocystine) and indirect effects (e.g., reduction of glutathione peroxidase). In addition, it is estimated that as much as 50% of the cellular antioxidant glutathione is produced from homocysteine by conversion through the transsulfuration pathway. | ||
A second popular hypothesis suggests that eHcy affects the control of biologically important methylation reactions by causing a build-up of S-adenosyl-L-homocysteine (AdoHcy) which is a competitive inhibitor of S-adenosyl-L-methionine (AdoMet) binding for methyltransferase enzymes. As methyltransferases are involved in a variety of important biological processes, inhibition of this class of enzymes could have extremely diverse effects on the organism.<ref>PMID:15890029</ref> | A second popular hypothesis suggests that eHcy affects the control of biologically important methylation reactions by causing a build-up of S-adenosyl-L-homocysteine (AdoHcy) which is a competitive inhibitor of S-adenosyl-L-methionine (AdoMet) binding for methyltransferase enzymes. As methyltransferases are involved in a variety of important biological processes, inhibition of this class of enzymes could have extremely diverse effects on the organism.<ref>PMID:15890029</ref> | ||
| + | |||
| + | ==3D structures of Cystathionine β-synthase== | ||
| + | |||
| + | [[Cystathionine β-synthase 3D structures]] | ||
| + | |||
</StructureSection> | </StructureSection> | ||
| + | |||
== Credits == | == Credits == | ||
Article created as an [https://is.cuni.cz/studium/predmety/index.php?id=b0728408dfab8c4e0250521ab9988dd8&tid=&do=predmet&kod=MB151P117&skr=2018 Structural biology of the cell] assignment at the Faculty of Science, Charles University, Prague, Czech Republic. | Article created as an [https://is.cuni.cz/studium/predmety/index.php?id=b0728408dfab8c4e0250521ab9988dd8&tid=&do=predmet&kod=MB151P117&skr=2018 Structural biology of the cell] assignment at the Faculty of Science, Charles University, Prague, Czech Republic. | ||
| Line 85: | Line 91: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | [[Category:Topic Page]] | ||
Current revision
3D Structure of Human Cystathionine β-synthase (4coo)
| |||||||||||
Credits
Article created as an Structural biology of the cell assignment at the Faculty of Science, Charles University, Prague, Czech Republic.
Assignment authors: Jana Křivková, Zdeňka Mauerová, Jan Hamalčík
References
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Oliveriusova J, Kraus JP, Burkhard P. Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):206-13. PMID:12686134
- ↑ Tu Y, Kreinbring CA, Hill M, Liu C, Petsko GA, McCune CD, Berkowitz DB, Liu D, Ringe D. Crystal Structures of Cystathionine beta-Synthase from Saccharomyces cerevisiae: One Enzymatic Step at a Time. Biochemistry. 2018 Apr 13. doi: 10.1021/acs.biochem.8b00092. PMID:29630349 doi:http://dx.doi.org/10.1021/acs.biochem.8b00092
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5'-phosphate-dependent heme protein. EMBO J. 2001 Aug 1;20(15):3910-6. PMID:11483494 doi:http://dx.doi.org/10.1093/emboj/20.15.3910
- ↑ Tu Y, Kreinbring CA, Hill M, Liu C, Petsko GA, McCune CD, Berkowitz DB, Liu D, Ringe D. Crystal Structures of Cystathionine beta-Synthase from Saccharomyces cerevisiae: One Enzymatic Step at a Time. Biochemistry. 2018 Apr 13. doi: 10.1021/acs.biochem.8b00092. PMID:29630349 doi:http://dx.doi.org/10.1021/acs.biochem.8b00092
- ↑ Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005 May-Jun;7(5-6):813-22. doi: 10.1089/ars.2005.7.813. PMID:15890029 doi:http://dx.doi.org/10.1089/ars.2005.7.813
- ↑ Tu Y, Kreinbring CA, Hill M, Liu C, Petsko GA, McCune CD, Berkowitz DB, Liu D, Ringe D. Crystal Structures of Cystathionine beta-Synthase from Saccharomyces cerevisiae: One Enzymatic Step at a Time. Biochemistry. 2018 Apr 13. doi: 10.1021/acs.biochem.8b00092. PMID:29630349 doi:http://dx.doi.org/10.1021/acs.biochem.8b00092
- ↑ Meier M, Oliveriusova J, Kraus JP, Burkhard P. Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):206-13. PMID:12686134
- ↑ Kraus JP, Janosik M, Kozich V, Mandell R, Shih V, Sperandeo MP, Sebastio G, de Franchis R, Andria G, Kluijtmans LA, Blom H, Boers GH, Gordon RB, Kamoun P, Tsai MY, Kruger WD, Koch HG, Ohura T, Gaustadnes M. Cystathionine beta-synthase mutations in homocystinuria. Hum Mutat. 1999;13(5):362-75. PMID:10338090 doi:<362::AID-HUMU4>3.0.CO;2-K http://dx.doi.org/10.1002/(SICI)1098-1004(1999)13:5<362::AID-HUMU4>3.0.CO;2-K
- ↑ Kozich V, Sokolova J, Klatovska V, Krijt J, Janosik M, Jelinek K, Kraus JP. Cystathionine beta-synthase mutations: effect of mutation topology on folding and activity. Hum Mutat. 2010 Jul;31(7):809-19. doi: 10.1002/humu.21273. PMID:20506325 doi:http://dx.doi.org/10.1002/humu.21273
- ↑ Meier M, Oliveriusova J, Kraus JP, Burkhard P. Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):206-13. PMID:12686134
- ↑ Meier M, Oliveriusova J, Kraus JP, Burkhard P. Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):206-13. PMID:12686134
- ↑ Kozich V, Sokolova J, Klatovska V, Krijt J, Janosik M, Jelinek K, Kraus JP. Cystathionine beta-synthase mutations: effect of mutation topology on folding and activity. Hum Mutat. 2010 Jul;31(7):809-19. doi: 10.1002/humu.21273. PMID:20506325 doi:http://dx.doi.org/10.1002/humu.21273
- ↑ Sperandeo MP, Candito M, Sebastio G, Rolland MO, Turc-Carel C, Giudicelli H, Dellamonica P, Andria G. Homocysteine response to methionine challenge in four obligate heterozygotes for homocystinuria and relationship with cystathionine beta-synthase mutations. J Inherit Metab Dis. 1996;19(3):351-6. PMID:8803779
- ↑ Mendes MI, Colaco HG, Smith DE, Ramos RJ, Pop A, van Dooren SJ, Tavares de Almeida I, Kluijtmans LA, Janssen MC, Rivera I, Salomons GS, Leandro P, Blom HJ. Reduced response of Cystathionine Beta-Synthase (CBS) to S-Adenosylmethionine (SAM): Identification and functional analysis of CBS gene mutations in Homocystinuria patients. J Inherit Metab Dis. 2014 Mar;37(2):245-54. doi: 10.1007/s10545-013-9647-6. Epub , 2013 Aug 23. PMID:23974653 doi:http://dx.doi.org/10.1007/s10545-013-9647-6
- ↑ Cui X, Navneet S, Wang J, Roon P, Chen W, Xian M, Smith SB. Analysis of MTHFR, CBS, Glutathione, Taurine, and Hydrogen Sulfide Levels in Retinas of Hyperhomocysteinemic Mice. Invest Ophthalmol Vis Sci. 2017 Apr 1;58(4):1954-1963. doi:, 10.1167/iovs.16-21247. PMID:28384716 doi:http://dx.doi.org/10.1167/iovs.16-21247
- ↑ Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014 Oct;10(4):281-8. doi: 10.3988/jcn.2014.10.4.281. Epub 2014, Oct 6. PMID:25324876 doi:http://dx.doi.org/10.3988/jcn.2014.10.4.281
- ↑ Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004 Jul 16;279(29):29871-4. Epub 2004 Apr 15. PMID:15087459 doi:http://dx.doi.org/10.1074/jbc.R400005200
- ↑ Meier M, Oliveriusova J, Kraus JP, Burkhard P. Structural insights into mutations of cystathionine beta-synthase. Biochim Biophys Acta. 2003 Apr 11;1647(1-2):206-13. PMID:12686134
- ↑ Jhee KH, Kruger WD. The role of cystathionine beta-synthase in homocysteine metabolism. Antioxid Redox Signal. 2005 May-Jun;7(5-6):813-22. doi: 10.1089/ars.2005.7.813. PMID:15890029 doi:http://dx.doi.org/10.1089/ars.2005.7.813