Nanog
From Proteopedia
m (User:Thiago Giove Mitsugi/Sandbox 1 moved to Nanog) |
|||
| (8 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
NANOG is a transcription factor, an upstream regulator in early mammalian development and a key determinant to maintain the pluripotency of embryonic stem cells. It’s named in reference to the Irish Legend of Oisín and Niamh and the Land of Eternal Youth, beauty, health and joy - Tír Na nÓg. [1] NANOG regulates the expression of hundreds of genes by binding their promoter elements. The murine Nanog homeodomain (HD) is capable of binding to interact with DNA elements derived from the Tcf3 promoter. [2] | NANOG is a transcription factor, an upstream regulator in early mammalian development and a key determinant to maintain the pluripotency of embryonic stem cells. It’s named in reference to the Irish Legend of Oisín and Niamh and the Land of Eternal Youth, beauty, health and joy - Tír Na nÓg. [1] NANOG regulates the expression of hundreds of genes by binding their promoter elements. The murine Nanog homeodomain (HD) is capable of binding to interact with DNA elements derived from the Tcf3 promoter. [2] | ||
The human NANOG protein coded by the NANOG1 gene, consists of 305 amino acids, molecular weight of 34.6 kDa and possesses 3 functional domains: the N-terminal domain, which contains 94 amino acids, is rich in Ser and Thr and acidic residues found in typical transactivators. The C-terminal domain contains 151 amino acids, it has a tryptophan repeat (WR) domain in which every fifth residue is a tryptophan. Last but not least, the conserved homeodomain motif has 60 amino acids. The homeodomain region facilitates not only DNA binding, optimally to the DNA consensus sequence 5'-TAAT[GT][GT]-3' or 5'-[CG][GA][CG]C[GC]ATTAN[GC]-3' [3], but also gene regulation and interaction with other proteins. The human Nanog 1 gene is located on chromosome 12, and the mRNA contains a 915 bp open reading frame (ORF) with 4 exons and 3 introns. [4][1]. | The human NANOG protein coded by the NANOG1 gene, consists of 305 amino acids, molecular weight of 34.6 kDa and possesses 3 functional domains: the N-terminal domain, which contains 94 amino acids, is rich in Ser and Thr and acidic residues found in typical transactivators. The C-terminal domain contains 151 amino acids, it has a tryptophan repeat (WR) domain in which every fifth residue is a tryptophan. Last but not least, the conserved homeodomain motif has 60 amino acids. The homeodomain region facilitates not only DNA binding, optimally to the DNA consensus sequence 5'-TAAT[GT][GT]-3' or 5'-[CG][GA][CG]C[GC]ATTAN[GC]-3' [3], but also gene regulation and interaction with other proteins. The human Nanog 1 gene is located on chromosome 12, and the mRNA contains a 915 bp open reading frame (ORF) with 4 exons and 3 introns. [4][1]. | ||
| + | |||
| + | == Structural highlights == | ||
| + | The <scene name='81/818528/Nanog/1'>struture of Nanog</scene> consists in 3 alpha helices linked to each other by loops. The so called alpha-helix1 and alpha-helix 3 play a role into binding of the protein to DNA. The alpha-helix1, localized in the N-terminal arm, binds to DNA through minor grooves, whereas alpha-helix3 binds major grooves in DNA and is considered the recognition helix, due to it´s extensive DNA contact sufarce. Alpha helices 1 and 2 are arrenged as antiparallel to each other in the terciary structure, while positioning alpha-Helix3 in a favorable way to bind to the major grooves of the DNA helical structure. | ||
| + | |||
| + | == DNA binding == | ||
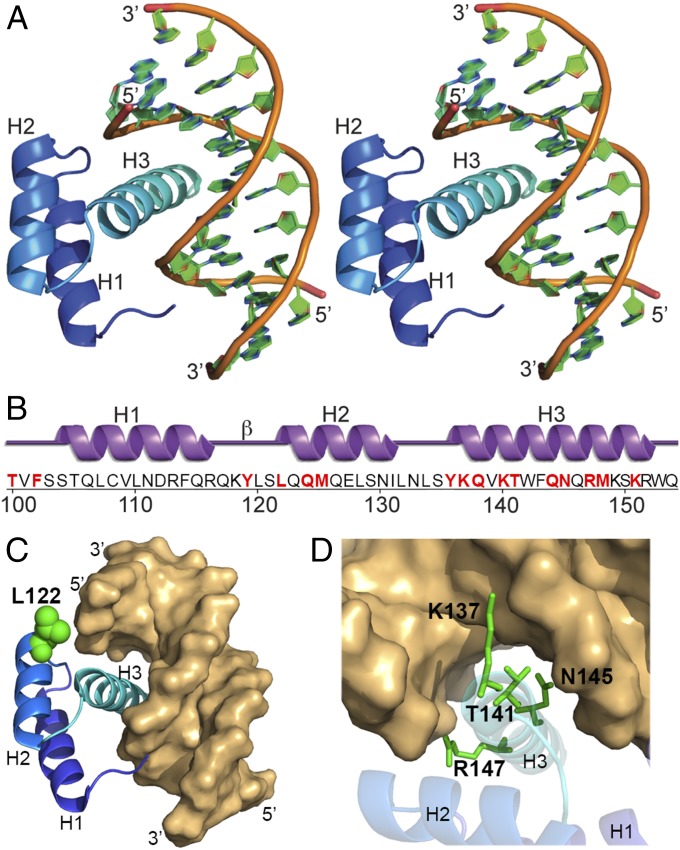
| + | Helix α3 (the so-called recognition helix) is inserted into the major groove of the DNA and forms an extensive DNA contact interface. The region upstream of helix α1 (HD residues 1–10) is referred to as the N-terminal arm and contributes to DNA binding through minor groove contacts. It was shown that in the absence and presence of DNA, the helical core of the HD constitutes a largely rigid entity that is not subjected to major structural changes upon binding to DNA. Pronounced flexibility is restricted to the N-terminal arm that becomes ordered after binding to the DNA's minor groove.[11][12] The figure below by Yohei Hayashia et al., 2015 shows more detail about the aminoacid residues participating in the binding between DNA and Nanog. | ||
| + | |||
| + | [[Image:Nanogg.jpg]] | ||
== Function == | == Function == | ||
| Line 16: | Line 24: | ||
| - | == Structural highlights == | ||
| - | The struture of Nanog consists in 3 alpha helices linked to each other by loops. The so called alpha-helix1 and alpha-helix 3 play a role into binding of the protein to DNA. The alpha-helix1, localized in the N-terminal arm, binds to DNA through minor grooves, whereas alpha-helix3 binds major grooves in DNA and is considered the recognition helix, due to it´s extensive DNA contact sufarce. Alpha helices 1 and 2 are arrenged as antiparallel to each other in the terciary structure, while positioning alpha-Helix3 in a favorable way to bind to the major grooves of the DNA helical structure. | ||
| - | |||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
| Line 42: | Line 46: | ||
10.Pan, Guangjin, and James A. Thomson. "Nanog and transcriptional networks in embryonic stem cell pluripotency." Cell research 17.1 (2007): 42. | 10.Pan, Guangjin, and James A. Thomson. "Nanog and transcriptional networks in embryonic stem cell pluripotency." Cell research 17.1 (2007): 42. | ||
| + | |||
| + | 11.Oh, J. H., Do, H. J., Yang, H. M., Moon, S. Y., Cha, K. Y., Chung, H. M. & Kim, J. H. (2005). Identification of a putative transactivation domain in human Nanog. Exp. Mol. Med. 37, 250–254. | ||
| + | |||
| + | 12.Frankel, A. D. (1992). The importance of being flexible. Proc. Natl Acad. Sci. USA, 89, 11653. | ||
Current revision
Nanog (Human homeobox protein)
| |||||||||||
References
1. Allouba, M. H., ElGuindy, A. M., Krishnamoorthy, N., Yacoub, M. H., & Aguib, Y. E. (2015). NaNog: A pluripotency homeobox (master) molecule. Global Cardiology Science and Practice, 2015(3), 36.doi:10.5339/gcsp.2015.36
2. Jauch, R. et al. Crystal Structure and DNA Binding of the Homeodomain of the Stem Cell Transcription Factor Nanog. J. Mol. Biol. (2008) 376, 758–770, doi:10.1016/j.jmb.2007.11.091
3. https://www.uniprot.org/uniprot/Q9H9S0
4. Gawlik-Rzemieniewska, N., & Bednarek, I. (2015). The role of NANOG transcriptional factor in the development of malignant phenotype of cancer cells. Cancer Biology & Therapy, 17(1), 1–10.doi:10.1080/15384047.2015.1121348
5. Zhang, W., Sui, Y., Ni, J., & Yang, T. (2016). Insights into the Nanog gene: A propeller for stemness in primitive stem cells. International Journal of Biological Sciences, 12(11), 1372–1381.doi:10.7150/ijbs.16349
6. Sun C, Sun L, Jiang K, et al. NANOG promotes liver cancer cell invasion by inducing epithelial-mesenchymal transition through NODAL/SMAD3 signaling pathway. The international journal of biochemistry & cell biology. 2013; 45:1099–1108. [PubMed: 23474366]
7. Chiou SH, Wang ML, Chou YT, et al. Coexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymaltransdifferentiation. Cancer Res. 2010; 70:10433–10444. [PubMed: 21159654].
8. Jeter CR, Liu B, Liu X, et al. NANOG promotes cancer stem cell characteristics and prostate cancer resistance to androgen deprivation. Oncogene. 2011; 30:3833–3845. [PubMed: 21499299]
9. Liu, Anfei, Xiya Yu, and Shanrong Liu. "Pluripotency transcription factors and cancer stem cells: small genes make a big difference." Chinese journal of cancer 32.9 (2013): 483.
10.Pan, Guangjin, and James A. Thomson. "Nanog and transcriptional networks in embryonic stem cell pluripotency." Cell research 17.1 (2007): 42.
11.Oh, J. H., Do, H. J., Yang, H. M., Moon, S. Y., Cha, K. Y., Chung, H. M. & Kim, J. H. (2005). Identification of a putative transactivation domain in human Nanog. Exp. Mol. Med. 37, 250–254.
12.Frankel, A. D. (1992). The importance of being flexible. Proc. Natl Acad. Sci. USA, 89, 11653.