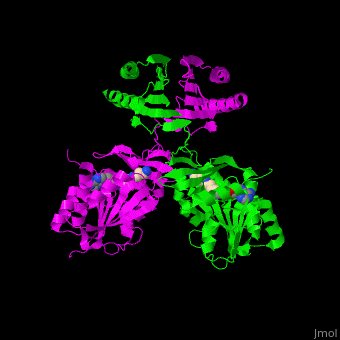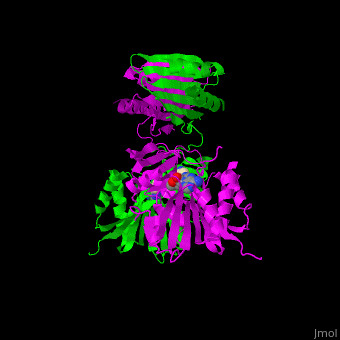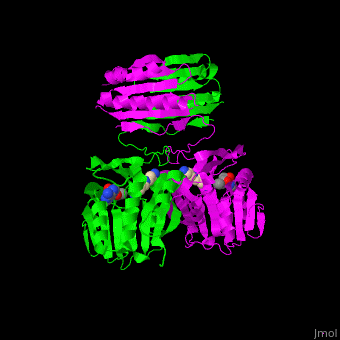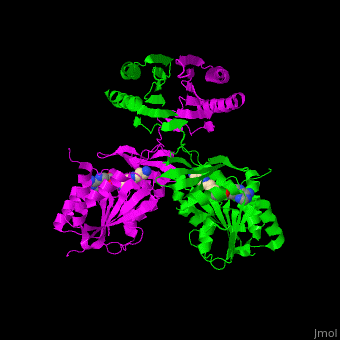We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Spermidine Synthase
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
== Function == | == Function == | ||
| - | Polyamines are essential in all branches of life. '''Spermidine synthase''' (putrescine aminopropyltransferase, PAPT) (SPS) catalyzes the biosynthesis of spermidine, a ubiquitous polyamine<ref>PMID:17585781</ref>. SPS catalyzes the transfer of aminopropyl group from decarboxylated S-adenosylmethionine (SAM) to the amine acceptor spermidine. | + | Polyamines are essential in all branches of life. '''Spermidine synthase''' (or '''putrescine aminopropyltransferase, polyamine aminopropyltransferase, PAPT''') (SPS) catalyzes the biosynthesis of spermidine, a ubiquitous polyamine<ref>PMID:17585781</ref>. SPS catalyzes the transfer of aminopropyl group from decarboxylated S-adenosylmethionine (SAM) to the amine acceptor spermidine. '''N4-Bis(aminopropyl) spermidine synthase''' or '''branched-chain polyamine synthase''' is an aminopropyltransferase essential for synthesis of branched-chain polyamines which enables thermophiles to grow in high temperature environments<ref>PMID:24610711</ref>. |
== Disease == | == Disease == | ||
| Line 9: | Line 9: | ||
==Structural highlights == | ==Structural highlights == | ||
<scene name='49/497058/Cv/7'>SPS active site contains the substrate spermidine</scene><ref>PMID:18367445</ref>. Water molecules are shown as red spheres. | <scene name='49/497058/Cv/7'>SPS active site contains the substrate spermidine</scene><ref>PMID:18367445</ref>. Water molecules are shown as red spheres. | ||
| - | + | ||
==3D structures of spermidine synthase== | ==3D structures of spermidine synthase== | ||
| + | [[Spermidine synthase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
Created with the participation of [[User:Lindsey Butler|Lindsey Butler]]. | Created with the participation of [[User:Lindsey Butler|Lindsey Butler]]. | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Wu H, Min J, Ikeguchi Y, Zeng H, Dong A, Loppnau P, Pegg AE, Plotnikov AN. Structure and mechanism of spermidine synthases. Biochemistry. 2007 Jul 17;46(28):8331-9. Epub 2007 Jun 22. PMID:17585781 doi:http://dx.doi.org/10.1021/bi602498k
- ↑ Okada K, Hidese R, Fukuda W, Niitsu M, Takao K, Horai Y, Umezawa N, Higuchi T, Oshima T, Yoshikawa Y, Imanaka T, Fujiwara S. Identification of a novel aminopropyltransferase involved in the synthesis of branched-chain polyamines in hyperthermophiles. J Bacteriol. 2014 May;196(10):1866-76. doi: 10.1128/JB.01515-14. Epub 2014 Mar 7. PMID:24610711 doi:http://dx.doi.org/10.1128/JB.01515-14
- ↑ Schwartz CE, Wang X, Stevenson RE, Pegg AE. Spermine synthase deficiency resulting in X-linked intellectual disability (Snyder-Robinson syndrome). Methods Mol Biol. 2011;720:437-45. doi: 10.1007/978-1-61779-034-8_28. PMID:21318891 doi:http://dx.doi.org/10.1007/978-1-61779-034-8_28
- ↑ Wu H, Min J, Zeng H, McCloskey DE, Ikeguchi Y, Loppnau P, Michael AJ, Pegg AE, Plotnikov AN. Crystal structure of human spermine synthase: implications of substrate binding and catalytic mechanism. J Biol Chem. 2008 Jun 6;283(23):16135-46. Epub 2008 Mar 26. PMID:18367445 doi:http://dx.doi.org/10.1074/jbc.M710323200
Created with the participation of Lindsey Butler.