We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Lipase
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
* The '''bile salt-stimulated lipase''' (BSSL) is found in breast milk.<br /> | * The '''bile salt-stimulated lipase''' (BSSL) is found in breast milk.<br /> | ||
* The '''hormone-sensitive lipase''' (LIPE) hydrolyzes a variety of esters. For details see [[Hormone sensitive lipase]].<br /> | * The '''hormone-sensitive lipase''' (LIPE) hydrolyzes a variety of esters. For details see [[Hormone sensitive lipase]].<br /> | ||
| - | * '''Monoacylglycerol lipase''' (MAGL) hydrolyzes intracellular triglycerides to fatty acid and glycerol. MAGL functions together with LIPE. For details see [[Monoglyceride lipase]]. | + | * '''Monoacylglycerol lipase''' (MAGL) hydrolyzes intracellular triglycerides to fatty acid and glycerol. MAGL functions together with LIPE. For details see [[Monoglyceride lipase]]. |
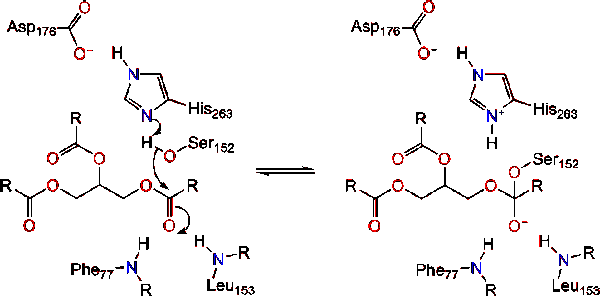
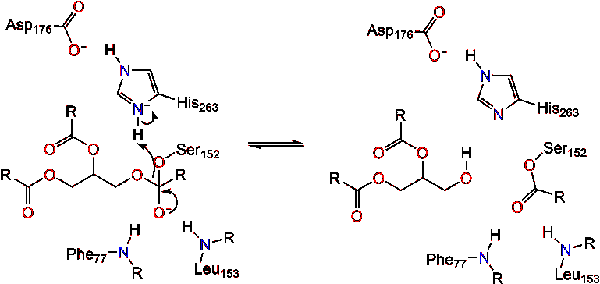
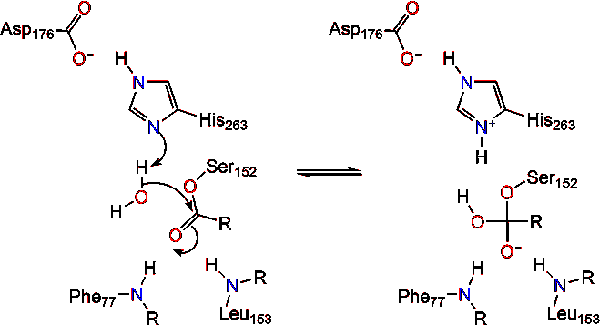
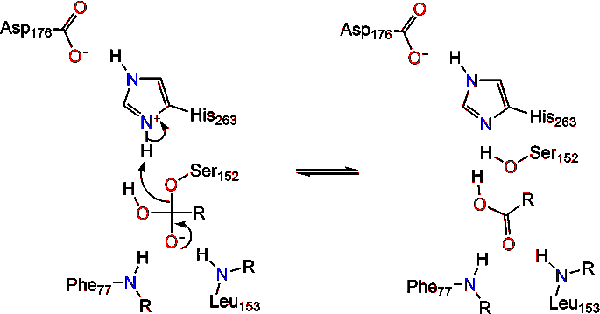
The reaction catalyzed by the enzyme is shown below. | The reaction catalyzed by the enzyme is shown below. | ||
| Line 13: | Line 13: | ||
[[Image:Picture 1.png]] | [[Image:Picture 1.png]] | ||
Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. An in depth discussion of the mechanism can be found in the Lipase Catalytic Mechanism section. The determination of the structure and function of lipase was a gradual process. Lipase activity was first demonstrated in the pancreas by Claude Bernard in 1846. However, it wasn't until 1955 that Mattson and Beck demonstrated a high-specificity of pancreatic lipase for triglyceride primary esters <ref name= "History of Lipids">[http://www.cyberlipid.org/history/history1.htm] History of Lipids</ref>. In recent years, determination of the crystal structure of pancreatic lipase has become the primary focus as many scientists have worked to further this.<br /> | Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. An in depth discussion of the mechanism can be found in the Lipase Catalytic Mechanism section. The determination of the structure and function of lipase was a gradual process. Lipase activity was first demonstrated in the pancreas by Claude Bernard in 1846. However, it wasn't until 1955 that Mattson and Beck demonstrated a high-specificity of pancreatic lipase for triglyceride primary esters <ref name= "History of Lipids">[http://www.cyberlipid.org/history/history1.htm] History of Lipids</ref>. In recent years, determination of the crystal structure of pancreatic lipase has become the primary focus as many scientists have worked to further this.<br /> | ||
| - | == | + | |
| + | ==See also== | ||
* [[Molecular Playground/Pancreatic Lipase]]<br /> | * [[Molecular Playground/Pancreatic Lipase]]<br /> | ||
* [[Lipase lid morph]]<br /> | * [[Lipase lid morph]]<br /> | ||
| Line 20: | Line 21: | ||
* [[Monoglyceride lipase]]<br /> | * [[Monoglyceride lipase]]<br /> | ||
* [[Human gastric lipase]]<br /> | * [[Human gastric lipase]]<br /> | ||
| + | * [[Lipoprotein Lipase (LPL) complexed with GPIHBP1]]<br /> | ||
* [[Lipase (Hebrew)]]<br /> | * [[Lipase (Hebrew)]]<br /> | ||
| + | * [[Lipid metabolism]] | ||
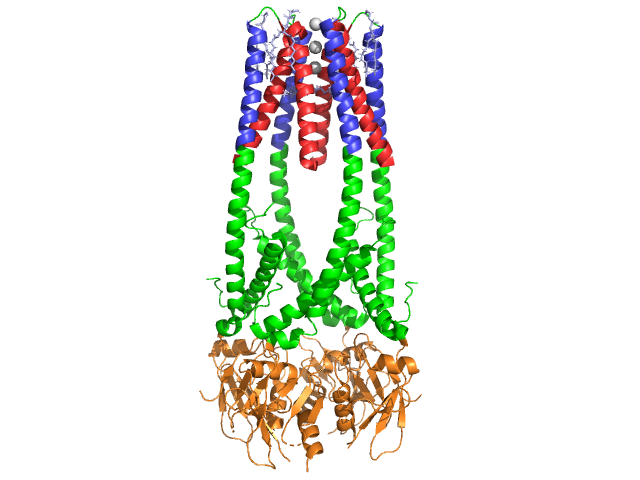
== '''Structure''' == | == '''Structure''' == | ||
Current revision
| |||||||||||
References
- ↑ [1] 1HPL PDB SUM
- ↑ [2] A cross-linked complex between horse pancreatic lipase and colipase
- ↑ [3] History of Lipids
- ↑ [4] 1HPL PDB
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL
- ↑ http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL
- ↑ http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf
- ↑ Fundamentals of Biochemistry...
- ↑ Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083.
- ↑ "Colipase". Wikipedia: The Free Encyclopedia. 5 July 2011 [5]
- ↑ "Colipase Residues..."
- ↑ Fundamentals of Biochemistry...
- ↑ Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512
- ↑ van Tilbeurgh H, etc."Structure of the pancreatic lipase-procolipase complex", 1992 Sep 10;359(6391):159-62. PMID:1522902.[6]
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1ETH
- ↑ http://www.nature.com/nature/journal/v362/n6423/abs/362814a0.html
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al.. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197-211. PMID:1409539
- ↑ Bourne Y, Martinez C, Kerfelec B, Lombardo D, Chapus C, Cambillau C. Horse pancreatic lipase. The crystal structure refined at 2.3 A resolution. J Mol Biol. 1994 May 20;238(5):709-32. PMID:8182745 doi:http://dx.doi.org/10.1006/jmbi.1994.1331
- ↑ [7] 1LPB PDB SUM
- ↑ "Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [8]
- ↑ Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Quinn R. Murray, Natalie Ziegler, Stephanie Schell, David Canner, Alexander Berchansky, Katelyn Clark, Eric Martz, Leben Tadesse, Joel L. Sussman, Eran Hodis