We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Succinate Dehydrogenase
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
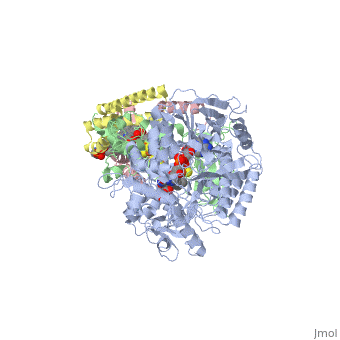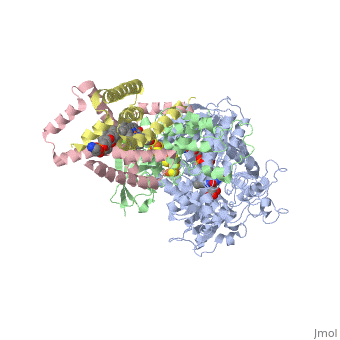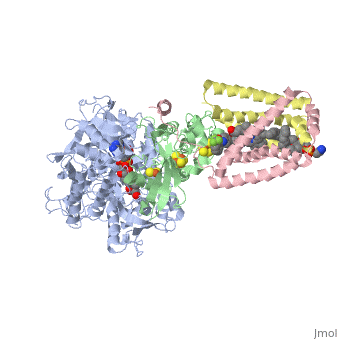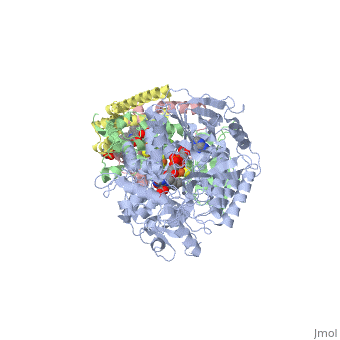
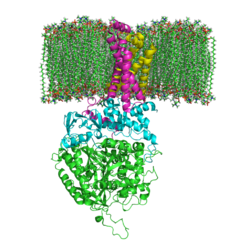
<StructureSection load='3ae1' size='350' side='right' caption='Succinate dehydrogenase containing flavoprotein subunit (Sdha) (grey), iron-sulfur subunit (Sdhb) (green), cytochrome B560 subunit (Sdhc) (pink) and cytochrome B small subunit (Sdhd) (yellow), FAD, Fe2S2, Fe4S4 and Fe3S4 complex with protoporphyrin, malonate, benzamide derivative and α-phosphatidyl-β-oleoyl-γ-palmitoyl-phosphatidylethanolamine, [[3ae1]] ' scene=''> | <StructureSection load='3ae1' size='350' side='right' caption='Succinate dehydrogenase containing flavoprotein subunit (Sdha) (grey), iron-sulfur subunit (Sdhb) (green), cytochrome B560 subunit (Sdhc) (pink) and cytochrome B small subunit (Sdhd) (yellow), FAD, Fe2S2, Fe4S4 and Fe3S4 complex with protoporphyrin, malonate, benzamide derivative and α-phosphatidyl-β-oleoyl-γ-palmitoyl-phosphatidylethanolamine, [[3ae1]] ' scene=''> | ||
[[Image:Succinate Dehydrogenase.png|left|250px]] | [[Image:Succinate Dehydrogenase.png|left|250px]] | ||
| - | + | {{Clear}} | |
== Function == | == Function == | ||
[[Succinate Dehydrogenase]] (PDB = [[2wdv]] with empty ubiquinone binding site; PDB = [[1nek]] with ubiquinone bound), also called '''succinate-coenzyme Q reductase''' (SQR) or '''Complex II''', is a tetrameric enzyme found in the cell membrane of http://proteopedia.org/wiki/index.php?title=Succinate_Dehydrogenase&action=editsome bacteria and the inner mitochondrial membrane of mammalian cells. It is classified as an α+β protein, as it contains <scene name='Michael_Vick_Sandbox_2/2wdv_sec_structure/1'>segregated regions</scene> of α helices and antiparallel β sheets. It is involved in two aspects of digestion; it catalyzes the oxidation of succinate to fumarate in the [[The_Citric_Acid_Cycle|citric acid cycle]] by simultaneously reducing ubiquinone to ubiquinol in the electron transport chain <ref>PMID:14672929</ref>. See also:<br /> | [[Succinate Dehydrogenase]] (PDB = [[2wdv]] with empty ubiquinone binding site; PDB = [[1nek]] with ubiquinone bound), also called '''succinate-coenzyme Q reductase''' (SQR) or '''Complex II''', is a tetrameric enzyme found in the cell membrane of http://proteopedia.org/wiki/index.php?title=Succinate_Dehydrogenase&action=editsome bacteria and the inner mitochondrial membrane of mammalian cells. It is classified as an α+β protein, as it contains <scene name='Michael_Vick_Sandbox_2/2wdv_sec_structure/1'>segregated regions</scene> of α helices and antiparallel β sheets. It is involved in two aspects of digestion; it catalyzes the oxidation of succinate to fumarate in the [[The_Citric_Acid_Cycle|citric acid cycle]] by simultaneously reducing ubiquinone to ubiquinol in the electron transport chain <ref>PMID:14672929</ref>. See also:<br /> | ||
| Line 10: | Line 10: | ||
*[[Citric Acid Cycle]] | *[[Citric Acid Cycle]] | ||
*[[Krebs cycle step 6]] | *[[Krebs cycle step 6]] | ||
| + | *[[Glyoxylate cycle]] | ||
==Structure== | ==Structure== | ||
| Line 26: | Line 27: | ||
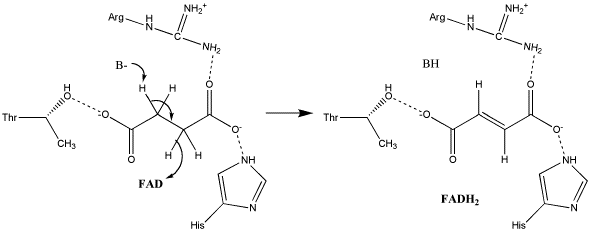
The exact mechanism for the oxidation of succinate to fumarate has not yet been elucidated. The initial deprotonation may be performed by FAD, Glu255, Arg286, or His242 of SdhA, and the following elimination may be a concerted E2 or E1cb elimination. In the concerted mechanism, the α-carbon is deprotonated by a base as FAD removes a hydride from the β-carbon; this is shown in image 1 <ref name="ghi">PMID:16950775</ref>. | The exact mechanism for the oxidation of succinate to fumarate has not yet been elucidated. The initial deprotonation may be performed by FAD, Glu255, Arg286, or His242 of SdhA, and the following elimination may be a concerted E2 or E1cb elimination. In the concerted mechanism, the α-carbon is deprotonated by a base as FAD removes a hydride from the β-carbon; this is shown in image 1 <ref name="ghi">PMID:16950775</ref>. | ||
| - | [[Image:S.D.Oxidation_of_Succinate_E2.gif|left| | + | [[Image:S.D.Oxidation_of_Succinate_E2.gif|left|600px]] |
{{Clear}} | {{Clear}} | ||
'''Image 1: Oxidation of succinate to fumarate through E2 elimination''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:S.D.Oxidation_of_Succinate_E2.gif). | '''Image 1: Oxidation of succinate to fumarate through E2 elimination''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:S.D.Oxidation_of_Succinate_E2.gif). | ||
| Line 32: | Line 33: | ||
In the proposed E1cb mechanism, the deprotonation leads to the formation of an enolate intermediate; FAD then removes the hydride, as shown in Image 2 <ref name="ghi" />. | In the proposed E1cb mechanism, the deprotonation leads to the formation of an enolate intermediate; FAD then removes the hydride, as shown in Image 2 <ref name="ghi" />. | ||
| - | [[Image:S.D.Oxidation_of_Succinate_E1cb.gif|left| | + | [[Image:S.D.Oxidation_of_Succinate_E1cb.gif|left|700px]] |
{{Clear}} | {{Clear}} | ||
'''Image 2: Oxidation of succinate to fumarate via E1cb elimination''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:S.D.Oxidation_of_Succinate_E1cb.gif). | '''Image 2: Oxidation of succinate to fumarate via E1cb elimination''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:S.D.Oxidation_of_Succinate_E1cb.gif). | ||
| Line 39: | Line 40: | ||
Ubiquinone is initially oriented in the active site such that the O1 carbonyl group interacts with Tyr83 of SdhD via hydrogen bonding. The electrons removed during the oxidation reaction are conveyed through the iron-sulfur clusters to 3Fe-4S; their presence on that cluster stimulates the substrate to reorient so that a second hydrogen bond between the <scene name='Michael_Vick_Sandbox_2/Ubq_binding_site/2'>O4 carbonyl group and Ser27</scene> of SdhC may form. The electrons are transferred to the substrate individually, with the addition of the first producing a radical semiquinone and the second completing the reduction to ubiquinol. This mechanism is illustrated in image 3 <ref name="ghi" />. | Ubiquinone is initially oriented in the active site such that the O1 carbonyl group interacts with Tyr83 of SdhD via hydrogen bonding. The electrons removed during the oxidation reaction are conveyed through the iron-sulfur clusters to 3Fe-4S; their presence on that cluster stimulates the substrate to reorient so that a second hydrogen bond between the <scene name='Michael_Vick_Sandbox_2/Ubq_binding_site/2'>O4 carbonyl group and Ser27</scene> of SdhC may form. The electrons are transferred to the substrate individually, with the addition of the first producing a radical semiquinone and the second completing the reduction to ubiquinol. This mechanism is illustrated in image 3 <ref name="ghi" />. | ||
| - | [[Image:QuinoneMechanism.gif|left| | + | [[Image:QuinoneMechanism.gif|left|700px]] |
{{Clear}} | {{Clear}} | ||
'''Image 3: Reduction of ubiquinone to ubiquinol''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:QuinoneMechanism.gif). | '''Image 3: Reduction of ubiquinone to ubiquinol''' (from Adamandalex in ''Wikimedia Commons'' http://en.wikipedia.org/wiki/File:QuinoneMechanism.gif). | ||
| Line 46: | Line 47: | ||
Since succinate dehydrogenase possesses multiple active sites that catalyze two different reactions, two classes of inhibitors function on the enzyme. The first class, which includes succinate analogs--both naturally-occuring TCA cycle intermediates like malate and oxaloacetate and the synthetic analog, malonate--contains some of the strongest succinate dehydrogenase inhibitors. The second class of inhibitors, which includes the ubiquinone analogs thenoyltrifluoroacetone and carboxin, binds to the ubiquinone active site and prevents reduction of the substrate<ref>PMID:17916065</ref>. | Since succinate dehydrogenase possesses multiple active sites that catalyze two different reactions, two classes of inhibitors function on the enzyme. The first class, which includes succinate analogs--both naturally-occuring TCA cycle intermediates like malate and oxaloacetate and the synthetic analog, malonate--contains some of the strongest succinate dehydrogenase inhibitors. The second class of inhibitors, which includes the ubiquinone analogs thenoyltrifluoroacetone and carboxin, binds to the ubiquinone active site and prevents reduction of the substrate<ref>PMID:17916065</ref>. | ||
| - | + | ||
==3D structures of succinate dehydrogenase== | ==3D structures of succinate dehydrogenase== | ||
| + | [[Succinate dehydrogenase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | **[[1nek]] – EcSDH flavoprotein + Fe-S protein + cytochrome B-556 + membrane anchor protein subunits + ubiquinone<br /> | ||
| - | **[[1nen]] - EcSDH flavoprotein + Fe-S protein + cytochrome B-556 + membrane anchor protein subunits + dinitrophenol-17<br /> | ||
| - | **[[2acz]] - EcSDH flavoprotein + Fe-S protein + cytochrome B-556 + membrane anchor protein subunits + atpenin<br /> | ||
| - | **[[2wdq]] - EcSDH flavoprotein + Fe-S protein + cytochrome B-556 + membrane anchor protein subunits + carboxin<br /> | ||
| - | **[[2wdr]] - EcSDH flavoprotein + Fe-S protein + cytochrome B-556 + membrane anchor protein subunits + pentachlorophenol<br /> | ||
| - | **[[3abv]], [[3ae1]], [[3ae2]], [[3ae3]], [[3ae4]], [[3ae5]], [[3ae6]], [[3ae7]], [[3ae8]], [[3ae9]], [[3aea]], [[3aeb]], [[3aec]], [[3aed]], [[3aeg]], [[4ytp]] - pSDH flavoprotein + Fe-S protein + cytochrome B subunits + benzamide derivative<br /> | ||
| - | **[[3aee]] - pSDH flavoprotein + Fe-S protein + cytochrome B subunits + atpenin<br /> | ||
| - | **[[2fbw]], [[2wqy]] - cSDH flavoprotein + IP + cytochrome B subunits + carboxin<br /> | ||
| - | **[[2h89]] - cSDH flavoprotein + IP + cytochrome B subunits + malonate<br /> | ||
| - | **[[3vr9]], [[4yxd]] - prSDH flavoprotein + Fe-S protein + cytochrome B subunits + flutolanil<br /> | ||
| - | **[[4ysx]] - prSDH flavoprotein + Fe-S protein + cytochrome B subunits + inhibitor<br /> | ||
| - | **[[4ysy]], [[4ysz]], [[4yt0]], [[4ytm]], [[4ytn]] - prSDH flavoprotein + Fe-S protein + cytochrome B subunits + benzamide derivative<br /> | ||
| - | **[[5c3j]], [[5c2t]] - prSDH flavoprotein + Fe-S protein + cytochrome B subunits + ubiquinone derivative<br /> | ||
| - | **[[3sfd]] - pSDH flavoprotein + Fe-S protein + cytochrome B subunits + oxalacetate + pentachlorophenol<br /> | ||
| - | **[[3sfe]] - pSDH flavoprotein + Fe-S protein + cytochrome B subunits + oxalacetate + thiabendazole<br /> | ||
| - | **[[1zp0]] - pSDH flavoprotein + Fe-S protein + cytochrome B subunits + thenoyltrifluoroactone + nitropropionate<br /> | ||
| - | **[[1yq3]] - cSDH flavoprotein + IP + cytochrome B subunits + ubiquinone + oxalacetate<br /> | ||
| - | **[[1yq4]] - cSDH flavoprotein + IP + cytochrome B subunits + ubiquinone + nitropropionate<br /> | ||
| - | **[[3vrb]] - prSDH flavoprotein + Fe-S protein + cytochrome B subunits + flutolanil + fumarate<br /> | ||
| - | }} | ||
==References== | ==References== | ||
<references/> | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Oyedotun KS, Lemire BD. The quaternary structure of the Saccharomyces cerevisiae succinate dehydrogenase. Homology modeling, cofactor docking, and molecular dynamics simulation studies. J Biol Chem. 2004 Mar 5;279(10):9424-31. Epub 2003 Dec 12. PMID:14672929 doi:10.1074/jbc.M311876200
- ↑ Tomitsuka E, Hirawake H, Goto Y, Taniwaki M, Harada S, Kita K. Direct evidence for two distinct forms of the flavoprotein subunit of human mitochondrial complex II (succinate-ubiquinone reductase). J Biochem. 2003 Aug;134(2):191-5. PMID:12966066
- ↑ 3.0 3.1 Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003 Jan 31;299(5607):700-4. PMID:12560550 doi:10.1126/science.1079605
- ↑ 4.0 4.1 Horsefield R, Yankovskaya V, Sexton G, Whittingham W, Shiomi K, Omura S, Byrne B, Cecchini G, Iwata S. Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction. J Biol Chem. 2006 Mar 17;281(11):7309-16. Epub 2005 Dec 27. PMID:16407191 doi:http://dx.doi.org/10.1074/jbc.M508173200
- ↑ Kenney WC. The reaction of N-ethylmaleimide at the active site of succinate dehydrogenase. J Biol Chem. 1975 Apr 25;250(8):3089-94. PMID:235539
- ↑ Voet, Donald, Charlotte W. Pratt, and Judith G. Voet. Fundamentals of Biochemistry: Life at the Molecular Level. 2nd Ed. Hoboken, NJ: Wiley, 2008.
- ↑ Vinogradov AD, Kotlyar AB, Burov VI, Belikova YO. Regulation of succinate dehydrogenase and tautomerization of oxaloacetate. Adv Enzyme Regul. 1989;28:271-80. PMID:2624174
- ↑ Boyd AW, Lackmann M. Signals from Eph and ephrin proteins: a developmental tool kit. Sci STKE. 2001 Dec 11;2001(112):re20. PMID:11741094 doi:10.1126/stke.2001.112.re20
- ↑ 9.0 9.1 9.2 Tran QM, Rothery RA, Maklashina E, Cecchini G, Weiner JH. The quinone binding site in Escherichia coli succinate dehydrogenase is required for electron transfer to the heme b. J Biol Chem. 2006 Oct 27;281(43):32310-7. Epub 2006 Sep 1. PMID:16950775 doi:10.1074/jbc.M607476200
- ↑ Muller FL, Liu Y, Abdul-Ghani MA, Lustgarten MS, Bhattacharya A, Jang YC, Van Remmen H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex I- and complex II-linked substrates. Biochem J. 2008 Jan 15;409(2):491-9. PMID:17916065 doi:10.1042/BJ20071162
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Michael Vick, David Canner, Alexander Berchansky