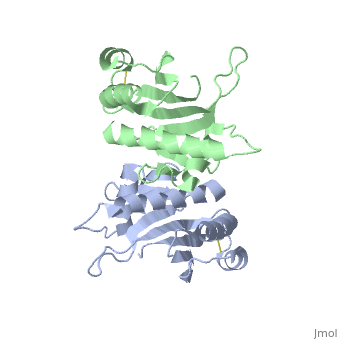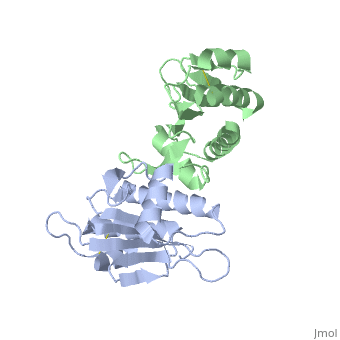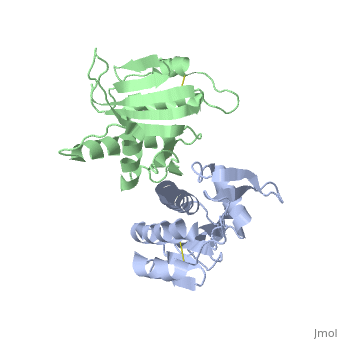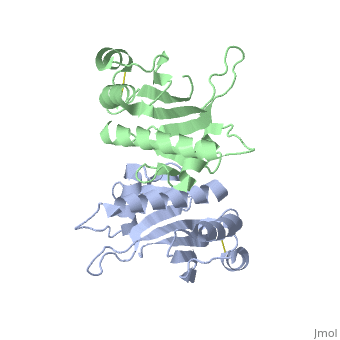We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Pilin
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 2: | Line 2: | ||
==Introduction== | ==Introduction== | ||
| - | '''Pilin''' (PIL) or '''fimbrial protein''' is a fibrous protein found in the pilus of bacteria. Pili in bacteria are used for exchange of DNA during bacterial conjugation. Type IV pili play an important role in the pathogenesis of many bacterial species; they are a required component for the adhesion of bacteria to their target cells. For example, Type IV pili are required for infection by the pathogens that cause cholera, typhoid, pneumonia, gonorrhea, and meningitis. <ref name=journal1>PMID:19626704</ref> They may also mediate transformation, modulate target-cell specificity and play a role in twitching motility. PilS is a protein that is a member of the pilin protein superfamily. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> | + | '''Pilin''' (PIL) or '''fimbrial protein''' is a fibrous protein found in the pilus of bacteria. Pili in bacteria are used for exchange of DNA during bacterial conjugation. '''Type IV pili''' play an important role in the pathogenesis of many bacterial species; they are a required component for the adhesion of bacteria to their target cells. For example, Type IV pili are required for infection by the pathogens that cause cholera, typhoid, pneumonia, gonorrhea, and meningitis. <ref name=journal1>PMID:19626704</ref> They may also mediate transformation, modulate target-cell specificity and play a role in twitching motility. PilS is a protein that is a member of the pilin protein superfamily. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> |
==Structure== | ==Structure== | ||
| - | Type IV pilin are homopolymers, composed of a single-chain pilin protein. The first common element that all type IVb pilins contain is the inclusion of the C-terminal β-strand (β7 in PilS) in the center of the structure, forming an antiparallel arrangement. <ref name=journal1>PMID:19626704</ref> A conserved 25 residue hydrophobic N terminal sequence, that serves as an oligomerization domain for fibre formation, is shared by all type IV pilins. Finally, all pilins also share a conserved pilus assembly machinery, a unique N-methylated N-terminus and a pair of conserved cysteines that form an intrachain disulphide bond. The type IVb pilus operon of ''S. typhi'' contains the piLS gene, which encodes a structural pilin. <ref name=journal1>PMID:19626704</ref> | + | Type IV pilin are homopolymers, composed of a single-chain pilin protein called '''major pilin''' and a less abundant protein called '''minor pilin''' which are essential for the pilus assembly and function<ref name=journal1>PMID:31784891</ref>. The first common element that all type IVb pilins contain is the inclusion of the C-terminal β-strand (β7 in PilS) in the center of the structure, forming an antiparallel arrangement. <ref name=journal1>PMID:19626704</ref> A conserved 25 residue hydrophobic N terminal sequence, that serves as an oligomerization domain for fibre formation, is shared by all type IV pilins. Finally, all pilins also share a conserved pilus assembly machinery, a unique N-methylated N-terminus and a pair of conserved cysteines that form an intrachain disulphide bond. The type IVb pilus operon of ''S. typhi'' contains the piLS gene, which encodes a structural pilin. <ref name=journal1>PMID:19626704</ref> |
The cysteine-containing region in the C-terminus is thought to play a function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> The D-region of the type IVb pilins is known to contain residues that function in pilus assembly; this region is stabilized by a conserved disulfide bond. <ref name=journal1>PMID:19626704</ref> | The cysteine-containing region in the C-terminus is thought to play a function via the formation of disulfides in CFTR adhesion and in the assembly of PilS to make the pilus of the bacterium. The highly conserved N-terminal hydrophobic tail functions as a oligomerization domain for fibre formation. <ref name=journal2>Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026</ref> The D-region of the type IVb pilins is known to contain residues that function in pilus assembly; this region is stabilized by a conserved disulfide bond. <ref name=journal1>PMID:19626704</ref> | ||
Current revision
| |||||||||||
Additional Resources
To See additional information, see: Bacterial Infections
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 Balakrishna AM, Saxena AM, Mok HY, Swaminathan K. Structural basis of typhoid: Salmonella typhi type IVb pilin (PilS) and cystic fibrosis transmembrane conductance regulator interaction. Proteins. 2009 Nov 1;77(2):253-61. PMID:19626704 doi:10.1002/prot.22500
- ↑ 2.0 2.1 2.2 Asha M. Balakrishna,Yvonne Yih-Wan Tan, Henry Yu-Keung Mok,Anand M. Saxena,and Kunchithapadam Swaminathan. Crystallization and preliminary X-ray diffraction analysis of Salmonella typhi PilS. 2006 October 1; 62(Pt 10): 1024–1026
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Elyse Yaremco, David Canner, Andrea Gorrell, Alexander Berchansky