We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Potassium Channel
From Proteopedia
(Difference between revisions)
(Undo revision 4316085 by Ann Taylor (Talk)) |
|||
| (5 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
==Function== | ==Function== | ||
| - | [[Potassium Channel]]'''s''' control cell membrane electric potentials by selectively allowing diffusion of K<sup>+</sup> across the membrane.<ref name="Zhou">PMID: 11689936</ref> K<sup>+</sup> Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.<ref name="Doyle">PMID: 9525859</ref> Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K<sup>+</sup> ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.<ref>PMID:12721618 | + | [[Potassium Channel]]'''s''' control cell membrane electric potentials by selectively allowing diffusion of K<sup>+</sup> across the membrane.<ref name="Zhou">PMID: 11689936</ref> K<sup>+</sup> Channels extend across the cell membrane, a 40Å thick lipid bilayer which ions cannot cross.<ref name="Doyle">PMID: 9525859</ref> Potassium homeostasis is crucial for nearly all living cells, but is particularly important for the correct function of neurons. Neurons produce electrical impulses known as action potentials, allow for cellular communication processes like neurotransmitter release or to initiate intercellular processes muscle contraction. At the onset of an action potential, sodium ions flood across the plasma membrane of neurons via sodium channels. The change in polarity of the plasma membrane caused by the sodium ion influx inactivates sodium channels. Potassium channels subsequently open allowing the selective diffusion of K<sup>+</sup> ions across the plasma membrane, returning the membrane polarity to neutral. After the action potential has passed, channels recreate the high potassium concentration within the cell in preparation for the next stiumulus.<ref>PMID:12721618</ref> |
Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K<sup>+</sup> ions without any outside energy expenditure. | Potassium channels possess two traits that are seemingly mutually exclusive. Firstly, potassium channels have exquisite selectivity, with an amazing 10,000 fold selectivity for K<sup>+</sup> ions over sodium ions. Considering the only difference by which potassium ions can be differentiated from sodium ions is potassium ions’ 1.33Å Pauling radius vs. Sodium’s .95Å radius, the selectivity of potassium channels is remarkable.<ref name="Doyle"/> Second, despite its remarkable selectivity, potassium channels allow for the transfer of K<sup>+</sup> ions across the cell membrane at a rate of nearly 10<sup>8</sup> per second, nearly at the diffusion rate limit.<ref name="Long">PMID: 18004376</ref> Potassium channels are able to achieve these remarkable feats due to its amazing structural architecture, which contains several features which not only can sense the voltage potential across a membrane, but also selectively ferry K<sup>+</sup> ions without any outside energy expenditure. | ||
| Line 9: | Line 9: | ||
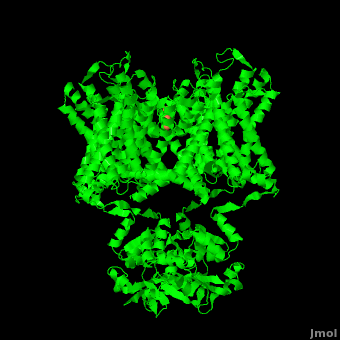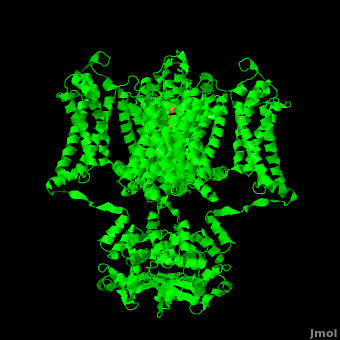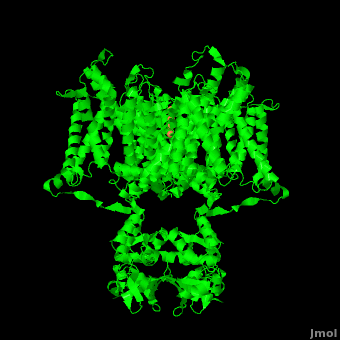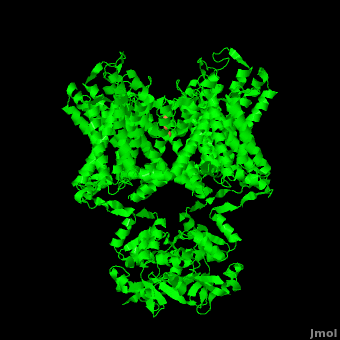
The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | The overall structure of the voltage gated potassium channel can be seen in the image at the left. It is comprised of 4 identical subunits and contains several key features which will be analyzed. Primarily, a <scene name='Potassium_Channel/Trans/3'>transmembrane region</scene> marked between the parallel lines in the figure. This region houses the <scene name='Potassium_Channel/Pore_opening/5'>channel pore</scene>, composed of interwoven helices in a teepee conformation, the all-important <scene name='Potassium_Channel/Selectivity_filter_opening/2'>“selectivity filter”</scene>, providing the channel with its remarkable 10,00 fold selectivity for K<sup>+</sup> ions over Na<sup>+</sup> ions and the <scene name='Potassium_Channel/Voltage_sensors_opening/4'>“voltage sensor”</scene> which is uses well placed arginine and acidic residues to determine the membrane polarity and open/close the channel in response.<ref name="Long"/> | ||
| - | ====Selectivity Filter and Pore | + | ==Disease== |
| + | |||
| + | Mutations in voltage-gated potassium channel KCNC3 have been linked with [[Neurodevelopmental Disorders|neurodevelopmental disorders]] and neurodegeneration.<ref>PMID: 16501573</ref>. [[Amiodarone]] is a potassium channel blocker used in treatment of cardiac dysrhythmias. | ||
| + | |||
| + | ==Selectivity Filter and Pore== | ||
It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | It is instructive to follow the path of a potassium ion as it enters the cell through the <scene name='Potassium_Channel/Potassium_out/3'>potassium channel</scene>. Upon <scene name='Potassium_Channel/Into_pore/4'>entering the channel</scene>, the K<sup>+</sup> ion first comes into contact with the <scene name='Potassium_Channel/From_extra/4'>selectivity filter</scene>. The solved structure of the potassium channel by MacKinnon et al. revealed where the channels remarkable selectivity comes from. When entering the <scene name='Potassium_Channel/From_extra/3'>selectivity filter</scene>, K<sup>+</sup> ions are first dehydrated, shedding up to 8 waters. To stabilize these naked ions, <scene name='Potassium_Channel/Selectivity_side/1'>a number of carbonyl oxygens</scene> (<scene name='Potassium_Channel/Selectivity_side_labels/3'>Labels</scene>) bind the K<sup>+</sup> ions. The distance between K<sup>+</sup> ion and carbonyl oxygen is at <scene name='Potassium_Channel/Selectivity_side_size/1'>the perfect width</scene> to accommodate K<sup>+</sup> ions but not Na<sup>+</sup>, ions which are too small. If a Na<sup>+</sup> ion were to lose its water shell, the carbonyl oxygens could not successfully stabilize it in its naked form and thus it is energetically unfavorable for a Na<sup>+</sup> ion to enter the channel. There is room within the selectivity filter for <scene name='Potassium_Channel/Selectivity_side_four/1'>four potassium ions</scene>. This, as it turns out, is crucial as the presence of the positive cations in close proximity to one another effectively pushes the potassium ions through the filter via electrostatic forces. This helps explain how the potassium channel can have such a rapid turnover rate.<ref name="Doyle"/> Also, the <scene name='Potassium_Channel/Selectivity_side_polarity/2'>natural polarity of the helices</scene>, with the <scene name='Potassium_Channel/Selectivity_side_polarity/3'>carbonyl oxygens pointing down the pore</scene>, helps pull the positively charged ions through the channel quickly. Compared to the <scene name='Potassium_Channel/High_filter/1'>high-concentration channel</scene> ([[1k4c]]), when exposed to a low concentration of potassium, the channel assumes a <scene name='Potassium_Channel/Low_con/3'>"low concentration" conformation</scene> ([[1k4d]]) which is sealed shut via interactions with water molecules.<ref name="Zhou"/> | ||
The <scene name='Potassium_Channel/High_filter_broad/1'>selectivity filter</scene> only makes up the beginning of the <scene name='Potassium_Channel/Full_pore/5'>channel pore</scene>. With the exception of the selectivity filter, the pore lining is <scene name='Potassium_Channel/Full_pore_hdryo/2'>mainly hydrophobic</scene>. This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired. Immediately following the selectivity filter is an <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene> (<scene name='Potassium_Channel/Full_pore_h20_spin/1'>Spinning Model</scene>). K<sup>+</sup> ions, after passing through the filter, rehydrate in this cavity, helping overcome much of the energetic difficulty of having a positively charged cation within a hydrophobic membrane. At the bottom of the 34Å pore containing transmembrane region lies a number of <scene name='Potassium_Channel/High_filter_aromatic/2'>aromatic residues</scene> which help form a seal between the pore and the intracellular cytoplasm.<ref name="Doyle"/> | The <scene name='Potassium_Channel/High_filter_broad/1'>selectivity filter</scene> only makes up the beginning of the <scene name='Potassium_Channel/Full_pore/5'>channel pore</scene>. With the exception of the selectivity filter, the pore lining is <scene name='Potassium_Channel/Full_pore_hdryo/2'>mainly hydrophobic</scene>. This hydrophobic lining provides an inert surface over which the diffusing ion can slide unimpaired. Immediately following the selectivity filter is an <scene name='Potassium_Channel/Full_pore_h20/1'>aqueous cavity</scene> (<scene name='Potassium_Channel/Full_pore_h20_spin/1'>Spinning Model</scene>). K<sup>+</sup> ions, after passing through the filter, rehydrate in this cavity, helping overcome much of the energetic difficulty of having a positively charged cation within a hydrophobic membrane. At the bottom of the 34Å pore containing transmembrane region lies a number of <scene name='Potassium_Channel/High_filter_aromatic/2'>aromatic residues</scene> which help form a seal between the pore and the intracellular cytoplasm.<ref name="Doyle"/> | ||
| - | + | ==Voltage Sensor== | |
Channel pore opening is dependent on the membrane voltage, a characteristic that is “sensed” by the <scene name='Potassium_Channel/Voltage_sensors_opening/6'>voltage sensor</scene>. The voltage sensor is comprised of <scene name='Potassium_Channel/Voltage_helices/3'>six helices</scene>, S0, S1, S2, S3, S4, & S5. Negatively charged sensor residues are either located in the <scene name='Potassium_Channel/Voltage_external/3'>external cluster</scene>, consisting of Glu 183 (in the [[2r9r]] structure) and Glu 226, or in the <scene name='Potassium_Channel/Voltage_internal/4'>internal cluster</scene> consisting of Glu 154, Glu 236, and Asp 259. The external cluster is exposed to solvent while the internal cluster is buried. <scene name='Potassium_Channel/Voltage_phe/2'>Phenylalanine 233</scene> acts as a separator between the two clusters.<ref name="Long"/> The 7 <scene name='Potassium_Channel/Voltage_phe/3'>positively charged residues</scene> of the voltage sensor are located on the S4 helix. Lys 302 and Arg 305 <scene name='Potassium_Channel/Voltage_lower_pos/1'>form hydrogen bonds</scene> with the internal negative cluster while Arginines 287, 290, 293, 296 and 299 are <scene name='Potassium_Channel/Voltage_solvent/1'>exposed to the extracellular solution</scene> (<scene name='Potassium_Channel/Voltage_sensors_arginines_over/2'>Overview</scene>). When the voltage sensor is exposed to a strong negative electric field in the intracellular membrane, the positive gating charges shift inward with the α-carbon of Arg 290 coming in close proximity to Phe 233. This shift effectively squeezes the pore shut, closing the intracellular-extracellular pathway. For a comparison see: The <scene name='Potassium_Channel/Open/1'>Open</scene> Channel vs. The <scene name='Potassium_Channel/Closed/1'>Closed</scene> ([[1k4c]]) Channel.<ref name="Long"/> Or view the morph of the <scene name='Potassium_Channel/Morph/3'>channel opening and closing</scene> (<scene name='Potassium_Channel/Morph/4'>Cartoon Design</scene>). | Channel pore opening is dependent on the membrane voltage, a characteristic that is “sensed” by the <scene name='Potassium_Channel/Voltage_sensors_opening/6'>voltage sensor</scene>. The voltage sensor is comprised of <scene name='Potassium_Channel/Voltage_helices/3'>six helices</scene>, S0, S1, S2, S3, S4, & S5. Negatively charged sensor residues are either located in the <scene name='Potassium_Channel/Voltage_external/3'>external cluster</scene>, consisting of Glu 183 (in the [[2r9r]] structure) and Glu 226, or in the <scene name='Potassium_Channel/Voltage_internal/4'>internal cluster</scene> consisting of Glu 154, Glu 236, and Asp 259. The external cluster is exposed to solvent while the internal cluster is buried. <scene name='Potassium_Channel/Voltage_phe/2'>Phenylalanine 233</scene> acts as a separator between the two clusters.<ref name="Long"/> The 7 <scene name='Potassium_Channel/Voltage_phe/3'>positively charged residues</scene> of the voltage sensor are located on the S4 helix. Lys 302 and Arg 305 <scene name='Potassium_Channel/Voltage_lower_pos/1'>form hydrogen bonds</scene> with the internal negative cluster while Arginines 287, 290, 293, 296 and 299 are <scene name='Potassium_Channel/Voltage_solvent/1'>exposed to the extracellular solution</scene> (<scene name='Potassium_Channel/Voltage_sensors_arginines_over/2'>Overview</scene>). When the voltage sensor is exposed to a strong negative electric field in the intracellular membrane, the positive gating charges shift inward with the α-carbon of Arg 290 coming in close proximity to Phe 233. This shift effectively squeezes the pore shut, closing the intracellular-extracellular pathway. For a comparison see: The <scene name='Potassium_Channel/Open/1'>Open</scene> Channel vs. The <scene name='Potassium_Channel/Closed/1'>Closed</scene> ([[1k4c]]) Channel.<ref name="Long"/> Or view the morph of the <scene name='Potassium_Channel/Morph/3'>channel opening and closing</scene> (<scene name='Potassium_Channel/Morph/4'>Cartoon Design</scene>). | ||
| Line 26: | Line 30: | ||
| - | '''Potassium channels''' (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. MthK is a calcium-dependent KCh from ''Methanobacterium thermoautrophicum''. | + | '''Potassium channels''' (KCh) are subdivided into voltage-gated KCh and calcium-dependent KCh. The latter are subdivided into high- (BK, LKCa), intermediate- and small-conductance KCh (human SK1, rat SK2, SKCa). The T1 domain is a highly conserved N-terminal domain which is responsible for driving the tetramerization of the KCh α subunit. The inward rectifier KCh (IRK) passes current more easily in the inward direction. KCh is activated by [[Phosphatidylinositol bisphosphate (PIP2)]]. MthK is a calcium-dependent KCh from ''Methanobacterium thermoautrophicum''. |
== 3D structures of Potassium Channels== | == 3D structures of Potassium Channels== | ||
Current revision
| |||||||||||
Additional Resources
References
- ↑ 1.0 1.1 Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 2001 Nov 1;414(6859):43-8. PMID:11689936 doi:http://dx.doi.org/10.1038/35102009
- ↑ 2.0 2.1 2.2 2.3 Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998 Apr 3;280(5360):69-77. PMID:9525859
- ↑ Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003 May 1;423(6935):33-41. PMID:12721618 doi:http://dx.doi.org/10.1038/nature01580
- ↑ 4.0 4.1 4.2 4.3 Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007 Nov 15;450(7168):376-82. PMID:18004376 doi:http://dx.doi.org/10.1038/nature06265
- ↑ Waters MF, Minassian NA, Stevanin G, Figueroa KP, Bannister JP, Nolte D, Mock AF, Evidente VG, Fee DB, Muller U, Durr A, Brice A, Papazian DM, Pulst SM. Mutations in voltage-gated potassium channel KCNC3 cause degenerative and developmental central nervous system phenotypes. Nat Genet. 2006 Apr;38(4):447-51. Epub 2006 Feb 26. PMID:16501573 doi:ng1758
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Joel L. Sussman, Alexander Berchansky, Ann Taylor