This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1568
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 17: | Line 17: | ||
When you look at the <scene name='82/823092/Spacefill_lsda/1'>spacefill view</scene> of the protein dimer you see that the binding pocket accessibility is very restrictive. | When you look at the <scene name='82/823092/Spacefill_lsda/1'>spacefill view</scene> of the protein dimer you see that the binding pocket accessibility is very restrictive. | ||
| - | [[Image:spacefill hydrophobicity.png]][[Image:ligand hydrophobicity.png]] | ||
| - | <scene name='82/823092/Hydrophobic_spacefill/1'>Hydrophobicity-focused</scene> view of the protein. | + | This is a <scene name='82/823092/Hydrophobic_spacefill/1'>Hydrophobicity-focused</scene> view of the protein. Overall, there doesn't seem to be any dominant hydrophobic or hydrophillic regions of the protein. |
The <scene name='82/823092/Catalytic_triad/2'>catalytic triad</scene> of the binding site consists of Phe59, Tyr101, and Lys134 that interact with the 4-hydroxyphenyl portion of the substrate. The triad importance was tested with specific mutations. A F59H mutation led to 3% efficiency comparable to wildtype LsdA. A Y101F mutation led to 20% efficiency comparable to wildtype LsdA. And a K134M mutation showed no discernible lignostilbene cleavage activity <ref>PMID 31292192</ref>. | The <scene name='82/823092/Catalytic_triad/2'>catalytic triad</scene> of the binding site consists of Phe59, Tyr101, and Lys134 that interact with the 4-hydroxyphenyl portion of the substrate. The triad importance was tested with specific mutations. A F59H mutation led to 3% efficiency comparable to wildtype LsdA. A Y101F mutation led to 20% efficiency comparable to wildtype LsdA. And a K134M mutation showed no discernible lignostilbene cleavage activity <ref>PMID 31292192</ref>. | ||
| Line 27: | Line 26: | ||
== '''Energy Transformation''' == | == '''Energy Transformation''' == | ||
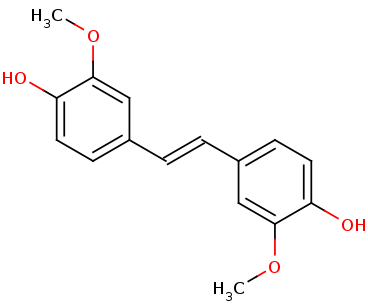
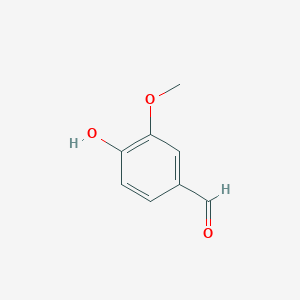
| - | + | LSDs are in the functional protein family called carotenoid cleavage oxygenases (CCOs). They are characterized by their cleavage of the double bond found in carotenoids. The binding site has ferrous iron supported by histidines and is kept in close proximity to the scissile double bond. There are two suggested mechanisms for LSD cleavage. McAndrew ''et al.'' suggested the hydroxystilbenoid is activated by enzyme-catalyzed deprotonation of the 4-hydroxy group. This causes electron delocalization toward the ferrous iron (-superoxo electrophile). The second proposed mechanism suggested by Sui ''et al.'' says that the pi bond electron density from the scissile double bond is redistributed to the iron-oxy complex to form an iron peroxo-substrate cation intermediate. In either case, deprotonation of the hydoxyl group is critical and assisted by the catalytic triad.<ref>PMID 31292192</ref> | |
| + | |||
| + | The enzyme serves to lower the activation energy and assist in the cleavage and transformation of the lignostilbene to two vanillins. | ||
</StructureSection> | </StructureSection> | ||
== '''References''' == | == '''References''' == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from Aug 26 through Dec 12, 2019 for use in the course CHEM 351 Biochemistry taught by Bonnie_Hall at the Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1556 through Sandbox Reserved 1575. |
To get started:
More help: Help:Editing |
Lignostilbene-α,ß-dioxygenase A structural features and important functional residues
| |||||||||||
References
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428
- ↑ Kuatsjah E, Verstraete MM, Kobylarz MJ, Liu AKN, Murphy MEP, Eltis LD. Identification of functionally important residues and structural features in a bacterial lignostilbene dioxygenase. J Biol Chem. 2019 Jul 10. pii: RA119.009428. doi: 10.1074/jbc.RA119.009428. PMID:31292192 doi:http://dx.doi.org/10.1074/jbc.RA119.009428