|
|
| (One intermediate revision not shown.) |
| Line 3: |
Line 3: |
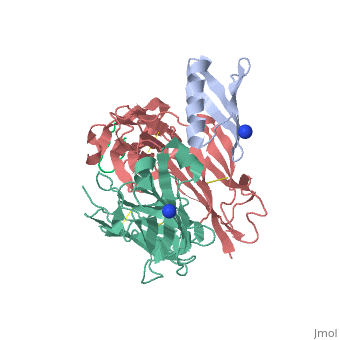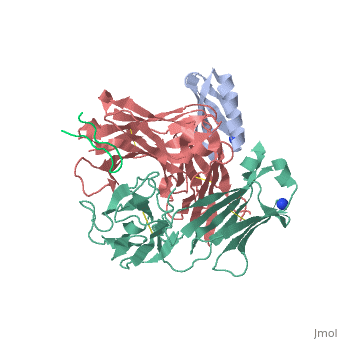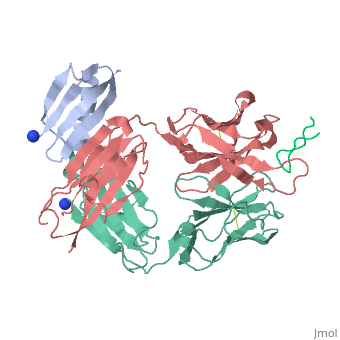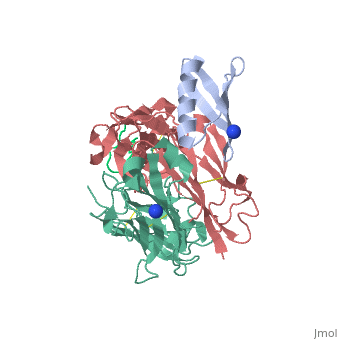
| | <StructureSection load='1uwx' size='340' side='right'caption='[[1uwx]], [[Resolution|resolution]] 2.20Å' scene=''> | | <StructureSection load='1uwx' size='340' side='right'caption='[[1uwx]], [[Resolution|resolution]] 2.20Å' scene=''> |
| | == Structural highlights == | | == Structural highlights == |
| - | <table><tr><td colspan='2'>[[1uwx]] is a 8 chain structure with sequence from [http://en.wikipedia.org/wiki/Mus_musculus Mus musculus] and [http://en.wikipedia.org/wiki/Strsp Strsp]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1UWX OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1UWX FirstGlance]. <br> | + | <table><tr><td colspan='2'>[[1uwx]] is a 8 chain structure with sequence from [https://en.wikipedia.org/wiki/Mus_musculus Mus musculus], [https://en.wikipedia.org/wiki/Neisseria_meningitidis Neisseria meningitidis] and [https://en.wikipedia.org/wiki/Streptococcus_sp. Streptococcus sp.]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1UWX OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=1UWX FirstGlance]. <br> |
| - | </td></tr><tr id='related'><td class="sblockLbl"><b>[[Related_structure|Related:]]</b></td><td class="sblockDat">[[1qkz|1qkz]]</td></tr> | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.2Å</td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1uwx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1uwx OCA], [http://pdbe.org/1uwx PDBe], [http://www.rcsb.org/pdb/explore.do?structureId=1uwx RCSB], [http://www.ebi.ac.uk/pdbsum/1uwx PDBsum], [http://prosat.h-its.org/prosat/prosatexe?pdbcode=1uwx ProSAT]</span></td></tr> | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=1uwx FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=1uwx OCA], [https://pdbe.org/1uwx PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=1uwx RCSB], [https://www.ebi.ac.uk/pdbsum/1uwx PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=1uwx ProSAT]</span></td></tr> |
| | </table> | | </table> |
| | + | == Function == |
| | + | [https://www.uniprot.org/uniprot/SPG2_STRSG SPG2_STRSG] |
| | == Evolutionary Conservation == | | == Evolutionary Conservation == |
| | [[Image:Consurf_key_small.gif|200px|right]] | | [[Image:Consurf_key_small.gif|200px|right]] |
| Line 30: |
Line 32: |
| | *[[Antibody 3D structures|Antibody 3D structures]] | | *[[Antibody 3D structures|Antibody 3D structures]] |
| | *[[Protein G|Protein G]] | | *[[Protein G|Protein G]] |
| | + | *[[3D structures of non-human antibody|3D structures of non-human antibody]] |
| | == References == | | == References == |
| | <references/> | | <references/> |
| Line 36: |
Line 39: |
| | [[Category: Large Structures]] | | [[Category: Large Structures]] |
| | [[Category: Mus musculus]] | | [[Category: Mus musculus]] |
| - | [[Category: Strsp]] | + | [[Category: Neisseria meningitidis]] |
| - | [[Category: Collins, R F]] | + | [[Category: Streptococcus sp]] |
| - | [[Category: Derrick, J P]] | + | [[Category: Collins RF]] |
| - | [[Category: Feavers, I M]] | + | [[Category: Derrick JP]] |
| - | [[Category: Maiden, M C.J]] | + | [[Category: Feavers IM]] |
| - | [[Category: Prince, S M]] | + | [[Category: Maiden MCJ]] |
| - | [[Category: Tzitzilonis, C]] | + | [[Category: Prince SM]] |
| - | [[Category: Antibody]]
| + | [[Category: Tzitzilonis C]] |
| - | [[Category: Antibody-complex]]
| + | |
| - | [[Category: Fab]]
| + | |
| - | [[Category: Igb]]
| + | |
| - | [[Category: Immune system]]
| + | |
| - | [[Category: Immunoglobulin]]
| + | |
| - | [[Category: Pora]]
| + | |
| - | [[Category: Protein g]]
| + | |
| Structural highlights
Function
SPG2_STRSG
Evolutionary Conservation
Check, as determined by ConSurfDB. You may read the explanation of the method and the full data available from ConSurf.
Publication Abstract from PubMed
Neisseria meningitidis is a globally important cause of bacterial meningitis and septicemia. No comprehensive antimeningococcal vaccine is available, largely as a consequence of the high sequence diversity of those surface proteins that could function as components of a vaccine. One such component is the protein PorA, a major surface porin of this Gram-negative organism that has been used in a number of experimental and licensed vaccines. Here we describe a series of experiments designed to investigate the consequences for antibody recognition of sequence diversity within a PorA antigen. The binding of a 14-residue peptide, corresponding to the P1.2 subtype antigen, to the MN16C13F4 monoclonal antibody was sensitive to mutation of five out of the six residues within the epitope sequence. The crystal structure of the antibody Fab fragment, determined in complex with the peptide antigen, shows a remarkably hydrophobic binding site and interactions between the antigen and antibody are dominated by apolar residues. Nine intrachain hydrogen bonds are formed within the antigen which maintain the beta-hairpin conformation of the peptide. These hydrogen bonds involve residues that are highly conserved amongst different P1.2 sequence variants, suggesting that some positions may be conserved for structural reasons in these highly polymorphic regions. The sensitivity of antibody recognition of the antigen towards mutation provides a structural explanation for the widespread sequence variation seen in different PorA sequences in this region. Single point mutations are sufficient to remove binding capability, providing a rationale for the manner in which different meningococcal PorA escape variants arise.
Structural variation and immune recognition of the P1.2 subtype meningococcal antigen.,Tzitzilonis C, Prince SM, Collins RF, Achtman M, Feavers IM, Maiden MC, Derrick JP Proteins. 2006 Mar 1;62(4):947-55. PMID:16470851[1]
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
See Also
References
- ↑ Tzitzilonis C, Prince SM, Collins RF, Achtman M, Feavers IM, Maiden MC, Derrick JP. Structural variation and immune recognition of the P1.2 subtype meningococcal antigen. Proteins. 2006 Mar 1;62(4):947-55. PMID:16470851 doi:http://dx.doi.org/10.1002/prot.20800
|