This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 1626
From Proteopedia
(Difference between revisions)
| (114 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{Sandbox_Reserved_CH462_Biochemistry_II}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | + | <scene name='83/832952/Tryptophan_proline/1'>Text To Be Displayed</scene>{{Sandbox_Reserved_CH462_Biochemistry_II}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
==Mitochondrial Calcium Uniporter (MCU) Complex== | ==Mitochondrial Calcium Uniporter (MCU) Complex== | ||
| - | <StructureSection load=' | + | <StructureSection load='6dnf' size='340' side='right' caption='Mitochondrial Calcium Uniporter (MCU): Each monomer of the MCU is shown in a different color. Additionally, glycerol molecules are shown in grey and red to indicate where the mitochondrial membrane exists. Calcium ions are shown in green. PDB 6dnf.' scene='83/832952/Starting_scene/1'> |
| - | + | ||
| - | + | ||
== Overview == | == Overview == | ||
| - | Calcium ions are universal and versatile signaling molecules. Their functions include muscle contraction, neuron excitability, and cell growth. Mitochondria regulate and decode calcium inputs that are necessary for many functions. Mitochondrial calcium regulates mitochondrial metabolism and has an impact in apoptosis, which will be talked about in greater depths later. This is important due to the fact an uncontrolled increase of calcium in the cytoplasm or prolonged presence of calcium in the mitochondria leads to apoptosis<ref name="Woods"/> The history behind the MCU came from the idea that individual mitochondria could take up high levels of calcium using ATP-derived energy founded in the 1960s.At resting conditions, the concentration of calcium in the mitochondria is around the same as in the cytoplasm (100-200 nM), but it can accumulate up to 10-20x that amount when stimulated. Calcium uptake into the mitochondrial matrix is driven by the membrane potential created by the electron transport chain. The calcium can flow through the outer membrane with ease as it is highly permeable, due to the pores formed by voltage-dependent anion-selective channel proteins. Now for the calcium pass through the inner membrane the MCU is needed. There are other pathways for calcium to get through the inner membrane but MCU is by far the most dominant. <ref name="Giorgi" /> | ||
| - | = | + | The mitochondrial calcium uniporter (MCU) complex is the main source of entry for [https://en.wikipedia.org/wiki/Calcium calcium] ions into the [https://en.wikipedia.org/wiki/Mitochondrial_matrix mitochondrial matrix] from the [https://en.wikipedia.org/wiki/Mitochondrion#Intermembrane_space intermembrane space]. MCU channels exist in most [https://en.wikipedia.org/wiki/Eukaryote eukaryotic] life, but activity is regulated differently in each [https://en.wikipedia.org/wiki/Clade clade].<ref name="Baradaran">PMID:29995857</ref> The precise identity of the MCU wasn't discovered until 2011 and was discovered using a combination of [https://en.wikipedia.org/wiki/Nuclear_magnetic_resonance_spectroscopy NMR spectroscopy], [https://en.wikipedia.org/wiki/Transmission_electron_cryomicroscopy cryo-electron microscopy], and [https://en.wikipedia.org/wiki/X-ray_crystallography x-ray crystallography].<ref name="Woods">PMID:31869674</ref> Identification of the structure was difficult because it has no apparent sequence similarity to other ion channels.<ref name="Baradaran"/> However, like other ion channels, it is incredibly selective and efficient. The MCU has the ability to only allow calcium ions into the mitochondrial matrix at a rate of 5,000,000 ions per second even though [https://en.wikipedia.org/wiki/Potassium potassium] ions are over 100,000 times more abundant in the intermembrane space.<ref name="Baradaran"/> |
| - | The precise identity of the MCU | + | |
| - | + | Under resting conditions, the calcium concentration in the mitochondria is about the same as in the [https://en.wikipedia.org/wiki/Cytoplasm cytoplasm], but when stimulated, it can increase calcium concentration 10-20-fold.<ref name="Giorgi">PMID:30143745</ref> Mitochondria-associated ER membranes ([https://en.wikipedia.org/wiki/Mitochondria_associated_membranes MAMs]) exist between mitochondria and the [https://en.wikipedia.org/wiki/Endoplasmic_reticulum endoplasmic reticulum], the two largest cellular stores of calcium, to allow for efficient transport of calcium ions.<ref name="Wang">PMID:28882140</ref> The transfer of electrons through respiratory complexes I-IV produces the energy to pump [https://en.wikipedia.org/wiki/Hydrogen_ion hydrogen ions] into the intermembrane space (IMS) and create the proton [https://en.wikipedia.org/wiki/Electrochemical_gradient electrochemical gradient] potential.<ref name="Giorgi"/> This negative electrochemical potential is the driving force that moves positively charged calcium ions into the mitochondrial matrix.<ref name="Giorgi"/> | |
| - | + | ||
| - | == | + | Regulation of the uptake and efflux of calcium is important to increase calcium levels enough to activate certain enzymes, but also avoid calcium overload and [https://en.wikipedia.org/wiki/Apoptosis apoptosis].<ref name="Wang"/> Mitochondrial calcium increases [http://proteopedia.org/wiki/index.php/ATP ATP] production by activating [http://proteopedia.org/wiki/index.php/Pyruvate_dehydrogenase pyruvate dehydrogenase], [https://en.wikipedia.org/wiki/Oxoglutarate_dehydrogenase_complex α-ketoglutarate dehydrogenase], and [http://proteopedia.org/wiki/index.php/Isocitrate_dehydrogenase isocitrate dehydrogenase] in the [https://en.wikipedia.org/wiki/Citric_acid_cycle Krebs cycle].<ref name="Wang"/> Therefore, deficiency of MCU leads to decrease of enzyme activity and of [https://en.wikipedia.org/wiki/Oxidative_phosphorylation oxidative phosphorylation]. |
| - | == | + | ==Structure== |
| - | + | ===Mitochondrial Calcium Uniporter Complex=== | |
| - | === | + | The mitochondrial calcium uniporter complex exists as a large complex (around 480 kDa in humans) made up of both pore-forming and regulatory subunits.<ref name="Wang"/> The mitochondrial uptake proteins (MICU1 and MICU2) are regulatory proteins in the MCU complex that exist in the IMS and contain [https://en.wikipedia.org/wiki/EF_hand EF hand domains] for calcium binding to control transport through the channel of the MCU complex.<ref name="Wang"/> When calcium ion concentration in the IMS is low, MICU1 and 2 block the MCU to prevent uptake of calcium.<ref name="Wang"/> In the presence of high calcium concentrations, more calcium binds to these regulatory proteins and they undergo a conformational change to allow calcium ions through the MCU and into the matrix.<ref name="Wang"/> In fact, when calcium levels are below 500 nM, MICU1 can block movement of calcium by itself, calcium levels between 500 nM and 1,500 nM require both MICU1 and MICU2 to block ion entry, and any concentration over 1,500 nM is sufficient for calcium entry.<ref name="Giorgi"/> Another regulatory protein, MCUR1 is a cofactor in the assembly of the [https://en.wikipedia.org/wiki/Electron_transport_chain respiratory chain] rather than an essential part of the uniporter.<ref name="Giorgi"/> Though the MCU is able to take up calcium independently, there are two other pore-forming subunits, the MCUb and the essential MCU regulator (EMRE).<ref name="Wang"/> MCUb is similar to MCU, but certain amino acids differ and make it an inhibitory subunit.<ref name="Wang"/> The EMRE is located in the IMS and connects MICU1 and MICU2 to the MCU.<ref name="Giorgi"/> It also contributes to regulation of calcium intake in the MCU.<ref name="Wang"/> |
| - | + | ===Mitochondrial Calcium Uniporter Structure=== | |
| - | + | The mitochondrial calcium uniporter (MCU) denotes the channel protein itself, not to be confused with the MCU complex. The MCU was originally thought to be composed of a [https://en.wikipedia.org/wiki/Pentamer pentamer] of five identical subunits, but it is now known to exist as a [https://en.wikipedia.org/wiki/Dimer_(chemistry) dimer] of <scene name='83/832952/Dimer_of_dimers/5'>dimers</scene>.<ref name="Woods">PMID:31869674</ref> The protein as a whole is composed of a [https://en.wikipedia.org/wiki/Coiled_coil coiled-coil] domain, a transmembrane domain, and a non-translated domain.<ref name="Woods"/> The hydrophobic <scene name='83/832952/New_ones/3'>transmembrane domain</scene> is located in the inner mitochondrial membrane ([https://en.wikipedia.org/wiki/Inner_mitochondrial_membrane IMM]) and the hydrophilic coiled-coil domain exists in the mitochondrial matrix.<ref name="Baradaran"/> The transmembrane domain (TMD) consists of eight separate helices (TM1 and TM2 from each subunit) that are connected by mostly hydrophobic amino acids in the IMS and has four-fold symmetry.<ref name="Baradaran"/> <scene name='83/832952/Tm1/2'>TM1</scene> packs tightly against <scene name='83/832952/Tm2/2'>TM2</scene> from the neighboring subunit which conveys a sense of domain-swapping.<ref name="Fan">PMID:29995856</ref> This section of the MCU can be roughly divided into a narrow outer leaflet portion with the selectivity filter and lined by the <scene name='83/832952/Tm2/2'>TM2</scene> helices and a wide inner leaflet.<ref name="Baradaran"/> Past the transmembrane domain, the N-terminal domains of the <scene name='83/832952/Tm1/2'>TM1</scene> helices extend into the matrix and form coiled-coils with a C-terminal helix.<ref name="Baradaran"/> These "legs" are separated from each other which allows enough space for calcium ions to diffuse out into the matrix.<ref name="Baradaran"/> Additionally, this domain is responsible for assembly of the MCU and [https://en.wikipedia.org/wiki/Post-translational_modification post-translational modification].<ref name="Fan"/> Finally, each leg ends in a <scene name='83/832952/New_ones/4'>non-translated domain</scene> (NTD).<ref name="Baradaran"/> While the MCU can intake calcium without the NTD, it may have regulatory functions and the ability to bend transmembrane helices to constrict the pore.<ref name="Baradaran"/> <ref name="Fan"/> | |
| - | === | + | ===Selectivity Filter=== |
| - | + | [[Image:Electronegativity_MCU_4.jpg|200 px|right|thumb|'''Fig. 1''' Electronegativity of the MCU viewed from outside the mouth of the channel. The high concentration of negative charge (shown in red) attracts the positive character of calcium ions. Created using PyMOL.]] | |
| - | + | The <scene name='83/832952/Selectivity_filter/3'>selectivity filter</scene> of the MCU is composed by many acidic amino acids near the narrow mouth of the channel which leads to high affinity for calcium ([https://en.wikipedia.org/wiki/Dissociation_constant dissociation constant] of less than 2nM).<ref name="Baradaran"/> The arrangement of the highly conserved <scene name='83/832952/Dxxe_motif/7'>WDXXEP</scene> [https://en.wikipedia.org/wiki/Sequence_motif motif] in the TM2 helices form a ring in the pore to which calcium ions are attracted.<ref name="Baradaran"/> The structure in the animation is the MCU of [http://www.mycobank.org/BioloMICS.aspx?Table=Mycobank&Rec=511257 ''Cyphellophora europaea''] so every amino acid named here specifically is that of ''C. europaea'', but most of these residues are highly conserved across all species, though residue number may change. Though not part of the <scene name='83/832952/Dxxe_motif/7'>WDXXEP</scene> motif, <scene name='83/832952/New_ones/2'>Asp221</scene> is present at the mouth of the MCU and serves to congregate positively charged <scene name='83/832952/Calcium/4'>calcium ions</scene> at the entrance of the channel.<ref name="Baradaran"/> The <scene name='83/832952/Dxxe_motif/7'>WDXXEP</scene> motif consists of <scene name='83/832952/Tryptophan/2'>Trp224</scene> at the N-terminal end, <scene name='83/832952/Selectivity_filter_asp/2'>Asp225</scene>, <scene name='83/832952/Selectivity_filter_glu/3'>Glu228</scene>, and <scene name='83/832952/New_ones/5'>Pro229</scene>.<ref name="Baradaran"/> <scene name='83/832952/Tryptophan_proline/2'>Trp224 and Pro229</scene> pack against each other and are oriented towards the pore, but only serve to stabilize <scene name='83/832952/Selectivity_filter_glu/4'>Asp225 and Glu228</scene>, not interact with calcium ions.<ref name="Baradaran"/><ref name="Fan"/> The X residues (<scene name='83/832952/New_ones/6'>Val226 and Met227</scene> in this case) face away from the pore and are exposed to the membrane.<ref name="Baradaran"/> The negatively charged side chains of Asp225 and Glu228 point towards the pore and form rings of radius 2.5Å and 1Å, respectively.<ref name="Baradaran"/> It's a combination of these radii and charges that account for the selectivity of the MCU. For example, potassium has an [https://en.wikipedia.org/wiki/Ionic_radius ionic radius] of 1.38Å which is much larger than the 1.00Å ionic radius of calcium and it therefore cannot fit into the negatively charged ring formed by Glu228.<ref name="Baradaran"/> Additionally, even though sodium ions have a similar ionic radius, the +2 charge on calcium is better matched to coordination with the glutamate residues.<ref name="Baradaran"/> | |
| - | + | ===Movement of Calcium=== | |
| - | === | + | Microscopy has revealed three sites in the MCU channel of roughly spherical density equally spaced 6Å apart.<ref name="Baradaran"/> Sites 1 and 2 lie within the selectivity filter so they can easily be assumed to contain calcium, but site 3 could be calcium or some other small molecule.<ref name="Baradaran"/> Site 1 is positioned in the ring formed by Asp225 residues and there is a distance of 4Å between the center of the site and each [https://en.wikipedia.org/wiki/Carboxylate carboxylate group] indicating presence of water.<ref name="Baradaran"/> The second site is positioned in the ring formed by Glu228 and there is a distance of 2.8Å between the carboxylate group of each residue and the middle of the site indicating absence of water.<ref name="Baradaran"/> It is hypothesized that one calcium ion coordinated with water in site 1 loses its water and moves to site 2 and a calcium ion moves from the IMS into site 1.<ref name="Baradaran"/> Meanwhile, a different calcium ion moves from site 2 to site 3 or the mitochondrial matrix.<ref name="Baradaran"/> |
| - | + | ===Mutations=== | |
| - | == | + | There are a number of mutations that completely eliminate calcium uptake by the MCU. For example, mutation of [https://en.wikipedia.org/wiki/Tryptophan W], [https://en.wikipedia.org/wiki/Aspartic_acid D], [https://en.wikipedia.org/wiki/Glutamic_acid E], or [https://en.wikipedia.org/wiki/Proline P] of the WDXXEP motif altered the highly conserved selectivity filter and completely eliminated calcium uptake.<ref name="Baradaran"/><ref name="Fan"/> For example, even mutating Glu228 to an aspartate significantly changed the dimensions of the pore and inhibited uptake of calcium.<ref name="Baradaran"/> However, mutation of either X residue was not detrimental to calcium uptake.<ref name="Baradaran"/> Furthermore, mutation of a tyrosine residue directly below the selectivity filter substantially impaired calcium intake and proper protein folding.<ref name="Fan"/> The residue on TM1 that affected calcium uptake the most in human MCU was Trp317 (analogous to <scene name='83/832952/New_ones/7'>Trp210</scene> in ''C. europaea'') which has a side chain constituting a primary contact point between TM1 and TM2.<ref name="Fan"/> Mutation of human MCU Phe326 (analogous to <scene name='83/832952/New_ones/8'>Phe218</scene> in ''C. europaea'') or Gly331 of the TM1-TM2 linker (<scene name='83/832952/New_ones/9'>Gly223</scene> in ''C. europaea'') affected the linker conformation and configuration of the pore entrance and impaired calcium intake.<ref name="Fan"/> |
| - | + | ==Regulation and Inhibition== | |
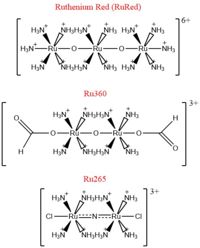
| - | + | [[Image:Ruthenium_Inhibitors.jpg|200 px|right|thumb|'''Fig. 2''' Structures of the ruthenium-based inhibitors of the MCU. Created using ChemDraw Professional 16.0]] | |
| - | + | The most well-known and commonly used inhibitor of the MCU is [https://en.wikipedia.org/wiki/Ruthenium_red ruthenium red] (RuRed).<ref name="Woods"/> RuRed is a trinuclear, oxo-bridged complex that effectively inhibits calcium uptake without affecting mitochondrial respiration or calcium efflux.<ref name="Woods"/> The disadvantage of ruthenium red is its challenging purification.<ref name="Woods"/> Interestingly, an impure version of RuRed, termed [https://en.wikipedia.org/wiki/Ru360 Ru360], was found to be the active component of RuRed and thus another good inhibitor of the MCU.<ref name="Woods"/> Ru360 is a binuclear, oxo-bridged complex with a similar structure to that of RuRed.<ref name="Woods"/> The only flaw with Ru360 was that it showed low cell permeability, so Ru265 was developed and had twice the cell permeability of Ru360.<ref name="Woods"/> Ru265 possesses two bridged Ru centers bridged by a nitride ligand.<ref name="Woods"/> | |
| - | + | Recent experiments suggest that Ru360 inhibits calcium uptake through interactions with the WDXXEP motif.<ref name="Woods"/> However, not much is actually known about the method of inhibition. Mutations of Asp261 and Ser259 in human MCU were shown to maintain calcium uptake into the matrix, but reduce the inhibitory effect of Ru360.<ref name="Woods"/> Curiously, the same Ser259 mutation did not affect inhibition of Ru265.<ref name="Woods"/> Additionally, a mutation in a cysteine residue in the NTD reduced the inhibitory effects of Ru265, but not Ru360.<ref name="Woods"/> So, while various inhibitors for the MCU are known, the mechanism of each is still largely unknown. | |
| - | + | ==Medical Relevance== | |
| - | </ | + | The MCU has a large role in disease because of its effect on apoptosis and cell signaling. The overload of the mitochondrial matrix with calcium leads to release of [https://en.wikipedia.org/wiki/Cytochrome_c cytochrome c], overproduction of [https://en.wikipedia.org/wiki/Reactive_oxygen_species reactive oxygen species], mitochondrial swelling, and the opening of the mitochondrial permeability transition pore ([https://en.wikipedia.org/wiki/Mitochondrial_permeability_transition_pore mPTP]) which all lead to apoptotic cell death.<ref name="Woods"/> This connection between mitochondrial calcium and apoptosis makes the MCU dysregulation a large contributor to cell death and disease. Calcium machinery in the mitochondria are targets for [https://en.wikipedia.org/wiki/Oncogene proto-oncogenes] and [https://en.wikipedia.org/wiki/Tumor_suppressor tumor suppressors] for this very reason.<ref name="Giorgi"/> Apoptosis can either be induced or repressed. Furthermore, external stimuli can activate receptors in the endoplasmic reticulum that release calcium and activate signal transductions.<ref name="Wang"/> Sequestration of calcium in the mitochondria is vital to shut down these activations, so any impact in movement of calcium ions can cause a wide variety of diseases.<ref name="Wang"/> |
| - | == | + | |
| - | + | ===Neurodegenerative Disorders=== | |
| - | <ref name=" | + | Disruption in calcium homeostasis leads to a wide range of [https://en.wikipedia.org/wiki/Neurodegeneration neurodegenerative disorders]. The MCU complex has been identified to play a large role in [https://en.wikipedia.org/wiki/Neuromuscular_disease neuromuscular disease] because of a loss or mutation of MICU1.<ref name="Woods"/> This causes [https://en.wikipedia.org/wiki/Myopathy myopathy], learning difficulties, and progressive movement disorders which can be lethal.<ref name="Woods"/> In [https://en.wikipedia.org/wiki/Alzheimer%27s_disease Alzheimer's disease], the buildup of [https://en.wikipedia.org/wiki/Amyloid_beta amyloid-β] plaques in the brain leads to increased calcium uptake in [https://en.wikipedia.org/wiki/Neuron neurons] and cell death.<ref name="Woods"/> Similarly, in early onset [https://en.wikipedia.org/wiki/Parkinson%27s_disease Parkinson's disease], degradation of MICU1 by the protein ligase [http://proteopedia.org/wiki/index.php/Parkin Parkin] leads to increased mitochondrial calcium uptake, overload, and death.<ref name="Woods"/> Finally, disrupted glutamate homeostasis in [https://en.wikipedia.org/wiki/Astrocyte astrocytes] and neurons leads to calcium overload and cell death via [https://en.wikipedia.org/wiki/Excitotoxicity excitotoxicity] in Amyotrophic Lateral Sclerosis ([https://en.wikipedia.org/wiki/Amyotrophic_lateral_sclerosis ALS]).<ref name="Woods"/> |
| - | + | ===Diabetes=== | |
| - | <ref name=" | + | Calcium homeostasis dysregulation has also been proven to be instrumental in [https://en.wikipedia.org/wiki/Obesity obesity], [https://en.wikipedia.org/wiki/Insulin_resistance insulin resistance], and [https://en.wikipedia.org/wiki/Type_2_diabetes type-II diabetes].<ref name="Wang"/> The intracellular calcium concentrations in primary [https://en.wikipedia.org/wiki/Adipocyte adipocytes] from obese human subjects has been found to be elevated.<ref name="Wang"/> Any inhbition of downstream calcium signaling could decrease movement of the [http://proteopedia.org/wiki/index.php/GLUT4 GLUT4] glucose transporter and glucose uptake.<ref name="Wang"/> Additionally, [https://en.wikipedia.org/wiki/Ablation ablation] of MCU in [https://en.wikipedia.org/wiki/Beta_cell β-cells] in the [https://en.wikipedia.org/wiki/Pancreas pancreas] demonstrated a decrease in cellular ATP concentration following glucose stimulation which resulted in decreased glucose-stimulated insulin secretion.<ref name="Wang"/> Furthermore, MAMs have been shown to cause glucose intolerance and mitochondrial dysfunction in primary [https://en.wikipedia.org/wiki/Hepatocyte hepatocytes] in mice.<ref name="Wang"/> Subsequent reinforcement of these MAMs has been shown to increase insulin sensitivity and glucose homeostasis.<ref name="Wang"/> |
| - | + | ||
| - | <ref name=" | + | |
| + | </StructureSection> | ||
| + | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | == Student Contributors == | ||
| + | Ryan Heumann | ||
| + | |||
| + | Rieser Wells | ||
Current revision
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Mitochondrial Calcium Uniporter (MCU) Complex
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0331-8. doi:, 10.1038/s41586-018-0331-8. PMID:29995857 doi:http://dx.doi.org/10.1038/s41586-018-0331-8
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Woods JJ, Wilson JJ. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr Opin Chem Biol. 2019 Dec 20;55:9-18. doi: 10.1016/j.cbpa.2019.11.006. PMID:31869674 doi:http://dx.doi.org/10.1016/j.cbpa.2019.11.006
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017 Sep 7;24(1):70. doi: 10.1186/s12929-017-0375-3. PMID:28882140 doi:http://dx.doi.org/10.1186/s12929-017-0375-3
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9
Student Contributors
Ryan Heumann
Rieser Wells