We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
5dfr
From Proteopedia
(Difference between revisions)
| Line 3: | Line 3: | ||
<StructureSection load='5dfr' size='340' side='right'caption='[[5dfr]], [[Resolution|resolution]] 2.30Å' scene=''> | <StructureSection load='5dfr' size='340' side='right'caption='[[5dfr]], [[Resolution|resolution]] 2.30Å' scene=''> | ||
== Structural highlights == | == Structural highlights == | ||
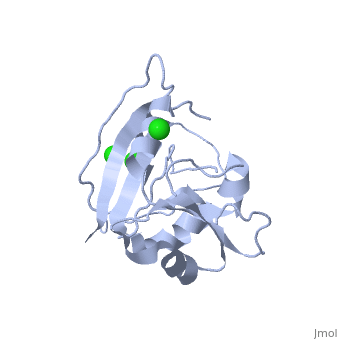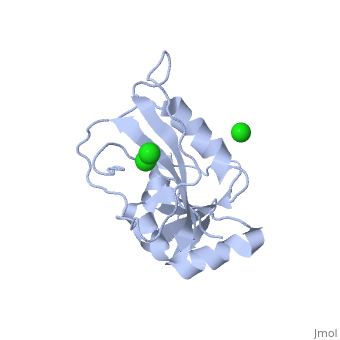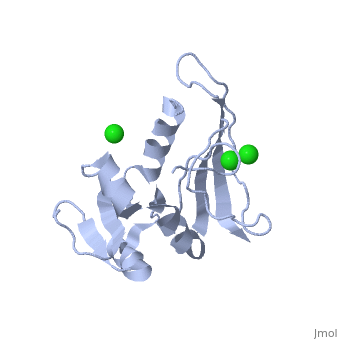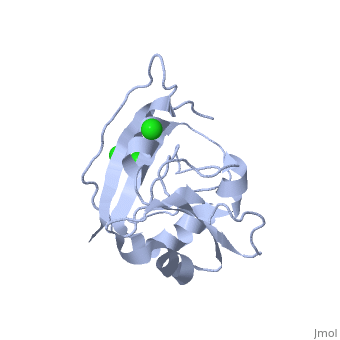
| - | <table><tr><td colspan='2'>[[5dfr]] is a 1 chain structure with sequence from [ | + | <table><tr><td colspan='2'>[[5dfr]] is a 1 chain structure with sequence from [https://en.wikipedia.org/wiki/Escherichia_coli Escherichia coli]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=5DFR OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=5DFR FirstGlance]. <br> |
| - | </td></tr><tr id=' | + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 2.3Å</td></tr> |
| - | <tr id=' | + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=CL:CHLORIDE+ION'>CL</scene></td></tr> |
| - | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[ | + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=5dfr FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=5dfr OCA], [https://pdbe.org/5dfr PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=5dfr RCSB], [https://www.ebi.ac.uk/pdbsum/5dfr PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=5dfr ProSAT]</span></td></tr> |
</table> | </table> | ||
== Function == | == Function == | ||
| - | [ | + | [https://www.uniprot.org/uniprot/DYR_ECOLI DYR_ECOLI] Key enzyme in folate metabolism. Catalyzes an essential reaction for de novo glycine and purine synthesis, and for DNA precursor synthesis. |
== Evolutionary Conservation == | == Evolutionary Conservation == | ||
[[Image:Consurf_key_small.gif|200px|right]] | [[Image:Consurf_key_small.gif|200px|right]] | ||
| Line 20: | Line 20: | ||
</jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=5dfr ConSurf]. | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=5dfr ConSurf]. | ||
<div style="clear:both"></div> | <div style="clear:both"></div> | ||
| - | <div style="background-color:#fffaf0;"> | ||
| - | == Publication Abstract from PubMed == | ||
| - | The crystal structure of unliganded dihydrofolate reductase (DHFR) from Escherichia coli has been solved and refined to an R factor of 19% at 2.3-A resolution in a crystal form that is nonisomorphous with each of the previously reported E. coli DHFR crystal structures [Bolin, J. T., Filman, D. J., Matthews, D. A., Hamlin, B. C., & Kraut, J. (1982) J. Biol. Chem. 257, 13650-13662; Bystroff, C., Oatley, S. J., & Kraut, J. (1990) Biochemistry 29, 3263-3277]. Significant conformational changes occur between the apoenzyme and each of the complexes: the NADP+ holoenzyme, the folate-NADP+ ternary complex, and the methotrexate (MTX) binary complex. The changes are small, with the largest about 3 A and most of them less than 1 A. For simplicity a two-domain description is adopted in which one domain contains the NADP+ 2'-phosphate binding site and the binding sites for the rest of the coenzyme and for the substrate lie between the two domains. Binding of either NADP+ or MTX induces a closing of the PABG-binding cleft and realignment of alpha-helices C and F which bind the pyrophosphate of the coenzyme. Formation of the ternary complex from the holoenzyme does not involve further relative domain shifts but does involve a shift of alpha-helix B and a floppy loop (the Met-20 loop) that precedes alpha B. These observations suggest a mechanism for cooperativity in binding between substrate and coenzyme wherein the greatest degree of cooperativity is expressed in the transition-state complex. We explore the idea that the MTX binary complex in some ways resembles the transition-state complex. | ||
| - | |||
| - | Crystal structure of unliganded Escherichia coli dihydrofolate reductase. Ligand-induced conformational changes and cooperativity in binding.,Bystroff C, Kraut J Biochemistry. 1991 Feb 26;30(8):2227-39. PMID:1998681<ref>PMID:1998681</ref> | ||
| - | |||
| - | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| - | </div> | ||
| - | <div class="pdbe-citations 5dfr" style="background-color:#fffaf0;"></div> | ||
==See Also== | ==See Also== | ||
*[[Dihydrofolate reductase 3D structures|Dihydrofolate reductase 3D structures]] | *[[Dihydrofolate reductase 3D structures|Dihydrofolate reductase 3D structures]] | ||
| - | *[[Molecular Playground/DHFR|Molecular Playground/DHFR]] | ||
| - | == References == | ||
| - | <references/> | ||
__TOC__ | __TOC__ | ||
</StructureSection> | </StructureSection> | ||
| - | [[Category: | + | [[Category: Escherichia coli]] |
| - | + | ||
[[Category: Large Structures]] | [[Category: Large Structures]] | ||
| - | [[Category: Bystroff | + | [[Category: Bystroff C]] |
| - | [[Category: Kraut | + | [[Category: Kraut J]] |
| - | + | ||
| - | + | ||
Current revision
CRYSTAL STRUCTURE OF UNLIGANDED ESCHERICHIA COLI DIHYDROFOLATE REDUCTASE. LIGAND-INDUCED CONFORMATIONAL CHANGES AND COOPERATIVITY IN BINDING
| |||||||||||