We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1601
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 5: | Line 5: | ||
== Overview == | == Overview == | ||
| - | + | [https://en.wikipedia.org/wiki/Insulin insulin] | |
| - | + | ||
| - | + | [[Image:Ruthenium_Red.jpg|300 px|right|thumb|'''Fig. 2''' Structure of Ruthenium Red]] | |
| - | + | ||
| - | + | ||
The mitochondrial calcium uniporter (MCU) complex is the main source of entry for calcium ions into the mitochondrial matrix from the intermembrane space. MCU channels exist in most eukaryotic life, but activity is regulated differently in each clade.<ref name="Baradaran">PMID:29995857</ref> The precise identity of the MCU wasn't discovered until 2011 and was discovered using a combination of NMR spectroscopy, cryo-electron microscopy, and x-ray crystallography.<ref name="Woods">PMID:31869674</ref> Identification of the structure was difficult because it has no apparent sequence similarity to other ion channels.<ref name="Baradaran"/> However, like other ion channels, it is incredibly selective and efficient. The MCU has the ability to only allow calcium ions into the mitochondrial matrix at a rate of 5,000,000 ions per second even though potassium ions are over 100,000 times more abundant in the intermembrane space.<ref name="Baradaran"/> | The mitochondrial calcium uniporter (MCU) complex is the main source of entry for calcium ions into the mitochondrial matrix from the intermembrane space. MCU channels exist in most eukaryotic life, but activity is regulated differently in each clade.<ref name="Baradaran">PMID:29995857</ref> The precise identity of the MCU wasn't discovered until 2011 and was discovered using a combination of NMR spectroscopy, cryo-electron microscopy, and x-ray crystallography.<ref name="Woods">PMID:31869674</ref> Identification of the structure was difficult because it has no apparent sequence similarity to other ion channels.<ref name="Baradaran"/> However, like other ion channels, it is incredibly selective and efficient. The MCU has the ability to only allow calcium ions into the mitochondrial matrix at a rate of 5,000,000 ions per second even though potassium ions are over 100,000 times more abundant in the intermembrane space.<ref name="Baradaran"/> | ||
| Line 15: | Line 13: | ||
Under resting conditions, the calcium concentration in the mitochondria is about the same as in the cytoplasm, but when stimulated, it can increase calcium concentration 10-20-fold.<ref name="Giorgi">PMID:30143745</ref> Mitochondria-associated ER membranes (MAMs) exist between mitochondria and the endoplasmic reticulum, the two largest cellular stores of calcium, to allow for efficient transport of calcium ions.<ref name="Wang">PMID:28882140</ref> The transfer of electrons through respiratory complexes I-IV produces the energy to pump hydrogen ions into the intermembrane space (IMS) and create the proton electrochemical gradient potential.<ref name="Giorgi"/> This negative electrochemical potential is the driving force that moves positively charged calcium ions into the mitochondrial matrix.<ref name="Giorgi"/> | Under resting conditions, the calcium concentration in the mitochondria is about the same as in the cytoplasm, but when stimulated, it can increase calcium concentration 10-20-fold.<ref name="Giorgi">PMID:30143745</ref> Mitochondria-associated ER membranes (MAMs) exist between mitochondria and the endoplasmic reticulum, the two largest cellular stores of calcium, to allow for efficient transport of calcium ions.<ref name="Wang">PMID:28882140</ref> The transfer of electrons through respiratory complexes I-IV produces the energy to pump hydrogen ions into the intermembrane space (IMS) and create the proton electrochemical gradient potential.<ref name="Giorgi"/> This negative electrochemical potential is the driving force that moves positively charged calcium ions into the mitochondrial matrix.<ref name="Giorgi"/> | ||
| - | Regulation of the uptake and efflux of calcium is important to increase calcium levels enough to activate certain enzymes, but also avoid calcium overload and apoptosis.<ref name="Wang"/> Mitochondrial calcium increases ATP production by activating pyruvate dehydrogenase, | + | Regulation of the uptake and efflux of calcium is important to increase calcium levels enough to activate certain enzymes, but also avoid calcium overload and apoptosis.<ref name="Wang"/> Mitochondrial calcium increases ATP production by activating pyruvate dehydrogenase, α-ketoglutarate dehydrogenase, and isocitrate dehydrogenase in the Krebs cycle.<ref name="Wang"/> Therefore, deficiency of MCU leads to decrease of enzyme activity and of oxidative phosphorylation. |
==Structure== | ==Structure== | ||
| Line 39: | Line 37: | ||
There are a number of mutations that completely eliminate calcium uptake by the MCU. For example, mutation of W,D,E, or P of the WDXXEP motif altered the highly conserved selectivity filter and completely eliminated calcium uptake.<ref name="Baradaran"/><ref name="Fan"/> For example, even mutating Glu228 to an aspartate significantly changed the dimensions of the pore and inhibited uptake of calcium.<ref name="Baradaran"/> However, mutation of either X residue was not detrimental to calcium uptake.<ref name="Baradaran"/> Furthermore, mutation of a tyrosine residue directly below the selectivity filter substantially impaired calcium intake and proper protein folding.<ref name="Fan"/> The residue on TM1 that affected calcium uptake the most in human MCU was Trp317 which has a side chain constituting a primary contact point between TM1 and TM2.<ref name="Fan"/> Mutation of Phe326 or Gly331 of the TM1-TM2 linker in human MCU affected the linker conformation and configuration of the pore entrance and impaired calcium intake.<ref name="Fan"/> | There are a number of mutations that completely eliminate calcium uptake by the MCU. For example, mutation of W,D,E, or P of the WDXXEP motif altered the highly conserved selectivity filter and completely eliminated calcium uptake.<ref name="Baradaran"/><ref name="Fan"/> For example, even mutating Glu228 to an aspartate significantly changed the dimensions of the pore and inhibited uptake of calcium.<ref name="Baradaran"/> However, mutation of either X residue was not detrimental to calcium uptake.<ref name="Baradaran"/> Furthermore, mutation of a tyrosine residue directly below the selectivity filter substantially impaired calcium intake and proper protein folding.<ref name="Fan"/> The residue on TM1 that affected calcium uptake the most in human MCU was Trp317 which has a side chain constituting a primary contact point between TM1 and TM2.<ref name="Fan"/> Mutation of Phe326 or Gly331 of the TM1-TM2 linker in human MCU affected the linker conformation and configuration of the pore entrance and impaired calcium intake.<ref name="Fan"/> | ||
| - | == | + | ==Regulation and Inhibition== |
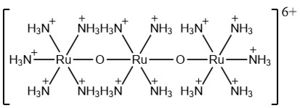
| - | === | + | The most well-known and commonly used inhibitor of the MCU is ruthenium red (RuRed).<ref name="Woods"/> RuRed is a trinuclear, oxo-bridged complex that effectively inhibits calcium uptake without affecting mitochondrial respiration or calcium efflux.<ref name="Woods"/> The disadvantage of ruthenium red is its challenging purification.<ref name="Woods"/> Interestingly, an impure version of RuRed, termed Ru360, was found to be the active component of RuRed and thus another good inhibitor of the MCU.<ref name="Woods"/> Ru360 is a binuclear, oxo-bridged complex with a similar structure to that of RuRed.<ref name="Woods"/> The only flaw with Ru360 was that it showed low cell permeability, so Ru265 was developed and had twice the cell permeability of Ru360.<ref name="Woods"/> Ru265 possesses two bridged Ru centers bridged by a nitride ligand.<ref name="Woods"/> |
| - | === | + | Recent experiments suggest that Ru360 inhibits calcium uptake through interactions with the WDXXEP motif.<ref name="Woods"/> However, not much is actually known about the method of inhibition. Mutations of Asp261 and Ser259 in human MCU (analogous to Asp225 and Ser223 in ''C. europaea'') were shown to maintain calcium uptake into the matrix, but reduce the inhibitory effect of Ru360.<ref name="Woods"/> Curiously, the same Ser259 mutation did not affect inhibition of Ru265.<ref name="Woods"/> Additionally, a mutation in a cysteine residue in the NTD reduced the inhibitory effects of Ru265, but not Ru360.<ref name="Woods"/> So, while various inhibitors for the MCU are known, the mechanism of each is still largely unknown. |
| - | == | + | ==Medical Relevance== |
| - | == | + | The MCU has a large role in disease because of its effect on apoptosis and cell signaling. The overload of the mitochondrial matrix with calcium leads to release of cytochrome c, overproduction of reactive oxygen species, mitochondrial swelling, and the opening of the mitochondrial permeability transition pore (mPTP) which all lead to apoptotic cell death.<ref name="Woods"/> This connection between mitochondrial calcium and apoptosis makes the MCU dysregulation a large contributor to cell death and disease. Calcium machinery in the mitochondria are targets for proto-oncogenes and tumor suppressors for this very reason.<ref name="Giorgi"/> Apoptosis can either be induced or repressed. Furthermore, external stimuli can activate receptors in the endoplasmic reticulum that release calcium and activate signal transductions.<ref name="Wang"/> Sequestration of calcium in the mitochondria is vital to shut down these activations, so any impact in movement of calcium ions can cause a wide variety of diseases.<ref name="Wang"/> |
| - | + | ===Neurodegenerative Disorders=== | |
| - | + | Disruption in calcium homeostasis leads to a wide range of neurodegenerative disorders. The MCU complex has been identified to play a large role in neuromuscular disease because of a loss or mutation of MICU1.<ref name="Woods"/> This causes myopathy, learning difficulties, and progressive movement disorders which can be lethal.<ref name="Woods"/> In Alzheimer's disease, the buildup of amyloid-β plaques in the brain leads to increased calcium uptake in neurons and cell death.<ref name="Woods"/> Similarly, in early onset Parkinson's Disorder, degradation of MICU1 by the protein ligase Parkin leads to increased mitochondrial calcium uptake, overload, and death.<ref name="Woods"/> Finally, disrupted glutamate homeostasis in astrocytes and neurons leads to calcium overload and cell death via excitotoxicity in Amyotrophic Lateral Sclerosis (ALS).<ref name="Woods"/> | |
| + | |||
| + | ===Diabetes=== | ||
| - | + | Calcium homeostasis dysregulation has also been proven to be instrumental in obesity, insulin resistance, and type-II diabetes.<ref name="Wang"/> The intracellular calcium concentrations in primary adipocytes from obese human subjects was found to be elevated.<ref name="Wang"/> Any inhbition of downstream calcium signaling could decrease movement of the GLUT4 glucose transporter and glucose uptake.<ref name="Wang"/> Additionally, ablation of MCU in β-cells in the pancreas demonstrated a decrease in cellular ATP concentration following glucose stimulation which resulted in decreased glucose-stimulated insulin secretion.<ref name="Wang"/> Furthermore, MAMs have been shown to cause glucose intolerance and mitochondrial dysfunction in primary hepatocytes in mice.<ref name="Wang"/> Subsequent reinforcement of these MAMs has been shown to increase insulin sensitivity and glucose homeostasis.<ref name="Wang"/> | |
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from Jan 13 through September 1, 2020 for use in the course CH462 Biochemistry II taught by R. Jeremy Johnson at the Butler University, Indianapolis, USA. This reservation includes Sandbox Reserved 1598 through Sandbox Reserved 1627. |
To get started:
More help: Help:Editing |
Mitochondrial Calcium Uniporter (MCU) (Heumann Test Page)
| |||||||||||
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 1.25 1.26 1.27 Baradaran R, Wang C, Siliciano AF, Long SB. Cryo-EM structures of fungal and metazoan mitochondrial calcium uniporters. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0331-8. doi:, 10.1038/s41586-018-0331-8. PMID:29995857 doi:http://dx.doi.org/10.1038/s41586-018-0331-8
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 Woods JJ, Wilson JJ. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr Opin Chem Biol. 2019 Dec 20;55:9-18. doi: 10.1016/j.cbpa.2019.11.006. PMID:31869674 doi:http://dx.doi.org/10.1016/j.cbpa.2019.11.006
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Giorgi C, Marchi S, Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat Rev Mol Cell Biol. 2018 Nov;19(11):713-730. doi: 10.1038/s41580-018-0052-8. PMID:30143745 doi:http://dx.doi.org/10.1038/s41580-018-0052-8
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 4.13 4.14 4.15 4.16 4.17 Wang CH, Wei YH. Role of mitochondrial dysfunction and dysregulation of Ca(2+) homeostasis in the pathophysiology of insulin resistance and type 2 diabetes. J Biomed Sci. 2017 Sep 7;24(1):70. doi: 10.1186/s12929-017-0375-3. PMID:28882140 doi:http://dx.doi.org/10.1186/s12929-017-0375-3
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 5.7 Fan C, Fan M, Orlando BJ, Fastman NM, Zhang J, Xu Y, Chambers MG, Xu X, Perry K, Liao M, Feng L. X-ray and cryo-EM structures of the mitochondrial calcium uniporter. Nature. 2018 Jul 11. pii: 10.1038/s41586-018-0330-9. doi:, 10.1038/s41586-018-0330-9. PMID:29995856 doi:http://dx.doi.org/10.1038/s41586-018-0330-9