This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
User:Samantha Schneider/Sandbox1
From Proteopedia
< User:Samantha Schneider(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
<StructureSection load='1fie' size='340' side='right' caption='Caption for this structure' scene=''> | <StructureSection load='1fie' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | Factor XIII, also known as fibrin stimulating factor is a Zymogen found in he blood of humans along with some other animals. It is activated by Thrombin to Factor XIIIa which enables it to preform it's enzymatic functions of cross-linking fibrin as part of the coagulation cascade. It was first detected in 1944 by Robbins. It is also known as the Laki-Lorand factor after Kalman Laki and Lazlo Lorand who proposed its existence in 1948. Factor XIII is a <scene name='84/842930/Heterotetramer/1'>heterotetramer</scene> that consists of 2 enzymatic A peptides and 2 non-enzymatic B peptides. | + | Factor XIII, also known as fibrin stimulating factor is a Zymogen found in he blood of humans along with some other animals. It is activated by Thrombin to Factor XIIIa which enables it to preform it's enzymatic functions of cross-linking fibrin as part of the coagulation cascade <ref>Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)</ref>. It was first detected in 1944 by Robbins. It is also known as the Laki-Lorand factor after Kalman Laki and Lazlo Lorand who proposed its existence in 1948. Factor XIII is a <scene name='84/842930/Heterotetramer/1'>heterotetramer</scene> that consists of 2 enzymatic A peptides and 2 non-enzymatic B peptides. |
== Structural highlights == | == Structural highlights == | ||
| - | The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. The FXIIIA subunit is composed of 4 structural units: <scene name='84/842930/Beta_sandwhich/1'>Beta sandwich</scene>, core, <scene name='84/842930/B-barrel_1/1'>barrel-1</scene>, and barrel-2 domains. The A subunit has a 37 amino acid N-terminal activation peptide, this is cleaved by thrombin during FXIII activation to FXIIIa. The [https://en.wikipedia.org/wiki/Beta-sandwich beta sandwhich] consists of residues 38-184. The <scene name='84/842930/Activation/1'>activation peptide</scene> is the first 37 amino acids on the N-terminal of the A subunit. | + | The fibrin stabilization factor is a heterotetramer that circulates throughout the blood plasma as a 320 kda molecule. It consists of a dimer of A subunits and a dimer of B subunits. The FXIIIA subunit is composed of 4 structural units: <scene name='84/842930/Beta_sandwhich/1'>Beta sandwich</scene>, core, <scene name='84/842930/B-barrel_1/1'>barrel-1</scene>, and <scene name='84/842930/Barrel-1/1'>barrel-2</scene> domains. The A subunit has a 37 amino acid N-terminal activation peptide, this is cleaved by thrombin during FXIII activation to FXIIIa. The [https://en.wikipedia.org/wiki/Beta-sandwich beta sandwhich] consists of residues 38-184. The <scene name='84/842930/Activation/1'>activation peptide</scene> is the first 37 amino acids on the N-terminal of the A subunit<ref>Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.</ref>. |
FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains. The <scene name='84/842930/Sushi-1/1'>Sushi</scene> domains which are each composed of approximately 60 amino acids. The B subunit is known to have a protective role, but recent research has suggested that there may be a regulatory role as well. The Sushi domain's variable length loop region is shown to have a hydrophobic interaction with the N-terminal activation region of the A subunit. The variable length loop region of the sushi-1 domain is electrostatically neutral. The Cab1 site is always exposed and therefore is bound by a calcium ion. | FXIIIB subunits are glycoproteins. The B subunit is made up of ten [https://en.wikipedia.org/wiki/Sushi_domain sushi] domains. The <scene name='84/842930/Sushi-1/1'>Sushi</scene> domains which are each composed of approximately 60 amino acids. The B subunit is known to have a protective role, but recent research has suggested that there may be a regulatory role as well. The Sushi domain's variable length loop region is shown to have a hydrophobic interaction with the N-terminal activation region of the A subunit. The variable length loop region of the sushi-1 domain is electrostatically neutral. The Cab1 site is always exposed and therefore is bound by a calcium ion. | ||
| Line 15: | Line 15: | ||
== Function == | == Function == | ||
| - | Factor XIII is a [[tranglutaminase]] that circulates throughout the blood as a heterotetramer. The B subunits bind to the clot structure. When fibrin is present, thrombin cleaves the <scene name='84/842930/Sessile_bond/1'>bond</scene> between the A and B subunits and the R37-G38 peptide bond in the A subunit that reveals it's active enzymatic region at the N-terminus. Calcium ions further activate the A subunits through a change in shape. The calcium ions additionally dissociate the non-covalently bound B subunits. The remaining dimer of two active A subunits, FactorXIIIa, crosslinks fibrin by forming isopeptide bonds between glutamines and lysines within the fibrin | + | Factor XIII is a [[tranglutaminase]] that circulates throughout the blood as a heterotetramer. The B subunits bind to the clot structure. When fibrin is present, thrombin cleaves the <scene name='84/842930/Sessile_bond/1'>bond</scene> between the A and B subunits and the R37-G38 peptide bond in the A subunit that reveals it's active enzymatic region at the N-terminus. Calcium ions further activate the A subunits through a change in shape. The calcium ions additionally dissociate the non-covalently bound B subunits. The remaining dimer of two active A subunits, FactorXIIIa, crosslinks fibrin by forming isopeptide bonds between glutamines and lysines within the fibrin<sup>2</sup> The crosslinks make the clot more durable and more resistant to fibrinolysis due to premature enzymatic degradation. It additionally has been found to play a role in proper wound healing, carrying pregnancy to full term, and in the development of new blood vessels. |
| - | FXIIIa catalyzes the formation of Nε(y-glutamyl)lysine protein to protein side chain bridges within the clot network. Fibrin fibers become thinner and longer once stabilized by FXIIIa although this doe not affect the clot density in any way. The cross-linking dramatically increases the clot stability and resistance to degradation. There is a transamidation reaction between Gln and Lys residues of neighboring molecules. | + | FXIIIa catalyzes the formation of Nε(y-glutamyl)lysine protein to protein side chain bridges within the clot network <sup>2</sup>. Fibrin fibers become thinner and longer once stabilized by FXIIIa although this doe not affect the clot density in any way. The cross-linking dramatically increases the clot stability and resistance to degradation. There is a transamidation reaction between Gln and Lys residues of neighboring molecules<sup>1</sup>. |
== Disease == | == Disease == | ||
| - | Factor XIII deficiency is a genetic bleeding disorder. It is an autosomal recessive disease. It is rare with Iran having the leading number of cases in the world. Individuals with this disorder form clots normally, however the clots are unstable and typically degrade which results in long bleeding episodes. Most phenotypical changes in factor XIII deficiency are caused by mutations in the A subunits. | + | Factor XIII deficiency is a genetic bleeding disorder. It is an autosomal recessive disease. It is rare with Iran having the leading number of cases in the world. Individuals with this disorder form clots normally, however the clots are unstable and typically degrade which results in long bleeding episodes. Most phenotypical changes in factor XIII deficiency are caused by mutations in the A subunits<ref>https://rarediseases.org/rare-diseases/factor-xiii-deficiency/</ref>. |
The symptoms of Factor XIII deficiency vary but in 80% of cases appear after birth with a bleeding episode stemming from the umbilical stump. Bleeding can occur spontaneously or from various activities. Commonly associated symptoms include chronic nosebleeds, bleeding from the gums, discoloration of the skin, and hematomas. These individuals typically bruise easily and spontaneously. 30% of people experience spontaneous intracranial hemorrhages. In homozygous woman spontaneous recurrent miscarriages can occur. | The symptoms of Factor XIII deficiency vary but in 80% of cases appear after birth with a bleeding episode stemming from the umbilical stump. Bleeding can occur spontaneously or from various activities. Commonly associated symptoms include chronic nosebleeds, bleeding from the gums, discoloration of the skin, and hematomas. These individuals typically bruise easily and spontaneously. 30% of people experience spontaneous intracranial hemorrhages. In homozygous woman spontaneous recurrent miscarriages can occur. | ||
| Line 29: | Line 29: | ||
In Class I cases, which constitute the majority of FXIII deficiency cases, there is virtually no thrombin dependent transamidase activity. In class 2 cases there has been a Val34Leu mutation in the A subunits. The class II mutation leads to a two-fold increase in FXIII. This mutation has shown to have protective effects against thrombotic disease in its population. | In Class I cases, which constitute the majority of FXIII deficiency cases, there is virtually no thrombin dependent transamidase activity. In class 2 cases there has been a Val34Leu mutation in the A subunits. The class II mutation leads to a two-fold increase in FXIII. This mutation has shown to have protective effects against thrombotic disease in its population. | ||
| - | == | + | == Sequence Conservation == |
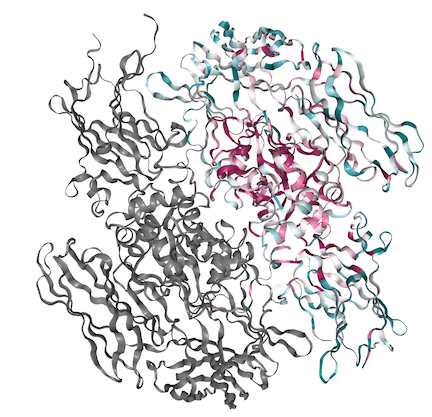
| + | [[Image:FXIIIstructure_conserved.png]] | ||
| + | |||
| + | |||
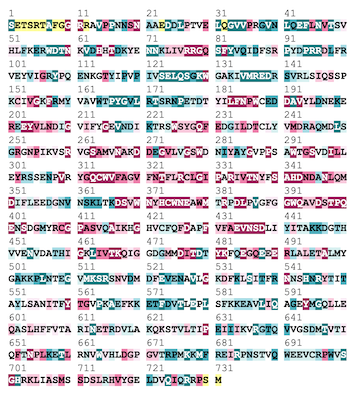
| + | Above is The A subunit fo FXIII shown with its conserved regions present in red and variable regions present in blue. Below is the coordinating sequence. As shown in the structure, a majority of the conserved region is located within the center of the molecule in the hydrophobic regions with variability present along the outside. | ||
| + | |||
| + | [[Image:FXIIIsequence.png]] | ||
| Line 38: | Line 44: | ||
[[Thrombin]] | [[Thrombin]] | ||
| + | |||
| + | [https://rarediseases.org/rare-diseases/factor-xiii-deficiency/ Factor XIII Deficiency] | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| - | 1.Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792. | ||
| - | |||
| - | 2. Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016). | ||
Current revision
Human Coagulation Factor XIII
| |||||||||||
References
- ↑ Gupta, S. et al. Revisiting the mechanism of coagulation factor XIII activation and regulation from a structure/functional perspective. Sci. Rep. 6, 30105; doi: 10.1038/srep30105 (2016)
- ↑ Muszbek L, Bereczky Z, Bagoly Z, Komáromi I, Katona É (July 2011). "Factor XIII: a coagulation factor with multiple plasmatic and cellular functions". Physiological Reviews. 91 (3): 931–72. doi:10.1152/physrev.00016.2010. PMID 21742792.
- ↑ https://rarediseases.org/rare-diseases/factor-xiii-deficiency/