We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Daniel Seeman
From Proteopedia
(Difference between revisions)
(Update current affiliation/title) |
m |
||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <center><span class="plainlinks">'''[linkedin.com/in/daniel-seeman Daniel Seeman, PhD]''' | + | <center><span class="plainlinks">'''[https://www.linkedin.com/in/daniel-seeman Daniel P. Seeman, PhD (Senior Scientist)]''' |
| Line 5: | Line 5: | ||
[[Image:delphiproteins.png|center|thumb|400px|Electrostatic potentials of three proteins (β-lactoglobulin, Bovine serum albumin, and Zn-Insulin) at pH 6. Calculated with DelPhi (a 'Nonlinear Poisson Boltzmann Solver') and displayed using UCSF Chimera. Protein charge anisotropy is a major component of both protein self-association, ''and'' interactions with bio-derived polyelectrolytes.]] | [[Image:delphiproteins.png|center|thumb|400px|Electrostatic potentials of three proteins (β-lactoglobulin, Bovine serum albumin, and Zn-Insulin) at pH 6. Calculated with DelPhi (a 'Nonlinear Poisson Boltzmann Solver') and displayed using UCSF Chimera. Protein charge anisotropy is a major component of both protein self-association, ''and'' interactions with bio-derived polyelectrolytes.]] | ||
| - | + | ||
Current revision
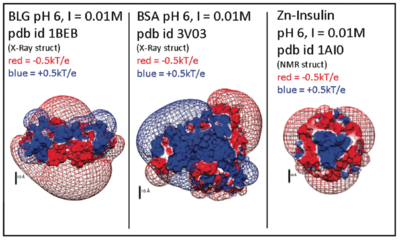
Electrostatic potentials of three proteins (β-lactoglobulin, Bovine serum albumin, and Zn-Insulin) at pH 6. Calculated with DelPhi (a 'Nonlinear Poisson Boltzmann Solver') and displayed using UCSF Chimera. Protein charge anisotropy is a major component of both protein self-association, and interactions with bio-derived polyelectrolytes.
About proteopedia:
- Topic Pages
