User:Andre Wu Le Chun/Sandbox 1
From Proteopedia
| (19 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
==Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up== | ==Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up== | ||
<StructureSection load='6vsb' size='340' side='right' caption='2019-nCoV spike glycoprotein with a single receptor-binding domain up. [[6vsb]]' scene=''> | <StructureSection load='6vsb' size='340' side='right' caption='2019-nCoV spike glycoprotein with a single receptor-binding domain up. [[6vsb]]' scene=''> | ||
| - | + | ||
| - | + | ||
== Structural highlights == | == Structural highlights == | ||
| - | + | <scene name='84/844931/6vsb/2'>6vsb</scene> is a homotrimer transmembrane glycoprotein(S) protruding from the viral surface. It is composed by two subunits that are fundamental for the virus entry in the cell: The S1 subunit, a v shaped subunit which contains the receptor binding domain (<scene name='84/844931/S_receptor-binding_domain/1'>RBD</scene>), which consists of the residues Arg319-Phe541, is responsible for binding to the host cell receptor, and the S2 subunit, which is responsible for fusion of the viral and cellular membranes. In order to allow the binding of the RBD in S1 with the host cell, the spike protein goes through conformational changes that make the binding domain assume a up conformation, thus, exposing the spike protein to its target receptor. | |
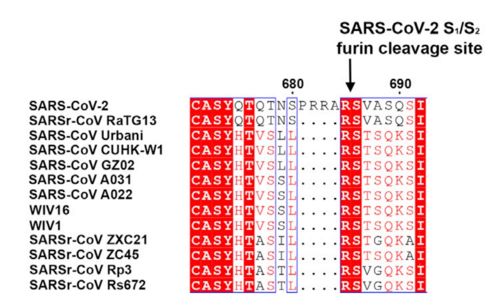
| - | This protein also shares amino acid sequence identity of 76% with SARS-CoV, however, as shown at the figure bellow, it has inserted amino acids that indicate a furin like cleavage site in the S1/S2 boundary. that must be primed in order to enable the viral pathogenicity. In the Receptor binding domain, units of beta-sheets, alpha-helices and loops can be observed. | ||
| + | This protein also shares amino acid sequence identity of 76% with SARS-CoV, however, as shown in the figure bellow, it has inserted amino acids that indicate a furin like cleavage site in the S1/S2 boundary. that must be primed in order to enable the viral pathogenicity. Regarding its secundary structure, | ||
| + | In the RBD, a β-sheet formed by 5 units of antiparallel β strands with units of α-helices and loops in between can be found. The receptor binding motif is located in a fragment that contains the β5 and β6 strands, α4 and α5 helices and loops. | ||
[[Image:Spike amino acids.jpg|500px|]] | [[Image:Spike amino acids.jpg|500px|]] | ||
| Line 22: | Line 22: | ||
== Disease == | == Disease == | ||
6vsb is probably the protein that enabled the Sars-Cov-2 to enter human cells, rusulting therefore on the large scale coronavirus epidemics in 2020. | 6vsb is probably the protein that enabled the Sars-Cov-2 to enter human cells, rusulting therefore on the large scale coronavirus epidemics in 2020. | ||
| - | + | The main symptoms of the COVID-19 were similar to the ones of common cold: fever, cough and tiredness, but with some stronger than the common cold: Chest pain, lost of smell and taste sense. | |
| - | The main reason for the worst symptoms | + | The main reason for the worst symptoms is the death of several types of cells in the lungs, causing severe loss of oxygenation, therefore, resulting of gradual colapse of the respiratory system of the infected. |
== Relevance == | == Relevance == | ||
| - | Due to its role in the infection process, the spike glyprotein may be a potential target of studies that seek methods of preventing | + | Due to its role in the infection process, the spike glyprotein may be a potential target of studies that seek methods of preventing COVID-19. These include the development of vaccines based on antibodies that are able to block conformational changes of the protein and its biding and fusion mechanisms. For instance, avoiding the cleavage of the furin, located on the B domain of the protein, by the host's proteases could be a way of viral inhibition. Comprehending how the protein can be bound to antibodies could also help the scientific community to prepare for SARS coronavirus outbreaks that may happen in the future. |
== Interaction with angiotensin-converting enzime 2 == | == Interaction with angiotensin-converting enzime 2 == | ||
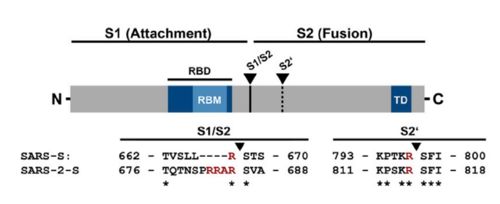
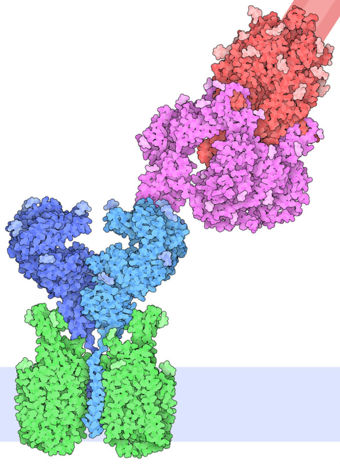
| - | The interaction between the 2019-nCov and the host cell begins with the recognition of the ACE2, a dimeric protein. Then, the S1 subunit moves, modifying the protein's conformation in way that receptor-binding domain becomes available to connect itself to the peptidase domain of | + | The interaction between the 2019-nCov and the host cell begins with the recognition of the ACE2, a dimeric protein. Then, the S1 subunit moves, modifying the protein's conformation in way that receptor-binding domain becomes available to connect itself to the peptidase domain of the receptor. At that moment, the spike protein is found in a "up" conformation. Due to the existence of four amino acids in between the 680 and 685 positions, a furin cleavage site is featured in the protein. Once this site is cleaved by host's furins, a later cleavage at S2' by a serine protease, TMPRSS2, happens in order to allow the activation of the fusion mechanism. Lastly, the spike protein makes a high affinity bond with ACE2 at the order of 15nM and a complex of spike protein and ACE2, as shown in figure 3, is formed. In order for the biding to happen 17 amino acid residues of the RBD interact with 20 amino acid residues of the receptor ACE2. It is important to consider the receptor binding domain similarity of both the SARS-CoV and the SARS-CoV-2. 14 amino acid positions are shared between the two conoraviruses RBD, these include Tyr449/Tyr436, Tyr453/Tyr440, Asn487/Asn473, Tyr489/Tyr475, Gly496/Gly482, Thr500/Thr486, Gly502/Gly488 and Tyr505/Tyr491, which are identical, and other 6 amino acid positions with altered residues. Also in the RBD, hydrophilic interctions such as 13 hydrogen bonds and 3 salt bridges can be found, tyrosine residues, which include Tyt449, Tyr489 and Tyr505, form hydrogen-bond interactions with polar hydroxil groups and in the complex formed by the spike protein and ACE2, a chain of Asn90-linked NAG-NAG-β-d-mannose, that may be useful for the bindin process can be found. |
| + | |||
[[Image:Spike.jpg|500px|]] | [[Image:Spike.jpg|500px|]] | ||
Figure 2: Alignment between S protein of SARS and SARS-2 (Hoffmann et al., 2020). | Figure 2: Alignment between S protein of SARS and SARS-2 (Hoffmann et al., 2020). | ||
| + | [[Image:SpikeACE2.jpg|340px|]] | ||
| + | Figure 3: Spike protein and ACE2 bound together (Goodsell, D., 2020) | ||
</StructureSection> | </StructureSection> | ||
| - | + | ||
| - | + | ||
| - | + | ||
<references/> | <references/> | ||
| + | Hoffmann, Markus & Kleine-Weber, Hannah & Schroeder, Simon & Krüger, Nadine & Herrler, Tanja & Erichsen, Sandra & Schiergens, Tobias & Herrler, Georg & Wu, Nai-Huei & Nitsche, Andreas & Müller, Marcel & Drosten, Christian & | ||
| + | |||
| + | Pöhlmann, Stefan. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 181. 10.1016/j.cell.2020.02.052. | ||
| + | |||
| + | Tortorici, M. Alejandra & Veesler, David. (2019). Structural insights into coronavirus entry. 10.1016/bs.aivir.2019.08.002. | ||
| + | |||
| + | Walls, Alexandra & Park, Young-Jun & Tortorici, M. & Wall, Abigail & Mcguire, Andrew & Veesler, David. (2020). Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. 10.1101/2020.02.19.956581. | ||
Current revision
6vsb
Prefusion 2019-nCoV spike glycoprotein with a single receptor-binding domain up
| |||||||||||
Hoffmann, Markus & Kleine-Weber, Hannah & Schroeder, Simon & Krüger, Nadine & Herrler, Tanja & Erichsen, Sandra & Schiergens, Tobias & Herrler, Georg & Wu, Nai-Huei & Nitsche, Andreas & Müller, Marcel & Drosten, Christian &
Pöhlmann, Stefan. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 181. 10.1016/j.cell.2020.02.052.
Tortorici, M. Alejandra & Veesler, David. (2019). Structural insights into coronavirus entry. 10.1016/bs.aivir.2019.08.002.
Walls, Alexandra & Park, Young-Jun & Tortorici, M. & Wall, Abigail & Mcguire, Andrew & Veesler, David. (2020). Structure, function and antigenicity of the SARS-CoV-2 spike glycoprotein. 10.1101/2020.02.19.956581.