User:Eric Martz/MHC Answers
From Proteopedia
(New page: ==Answers to Open-Ended Questions about MHC== Here are answers to the [http://molviz.org/antibody/MHC-questions.docx questions provided in a document] with the Major Histocompatibility Com...) |
(→Answers to Open-Ended Questions about MHC) |
||
| (15 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Answers to Open-Ended Questions about MHC== | ==Answers to Open-Ended Questions about MHC== | ||
| - | Here are answers to the [http://molviz.org/ | + | Here are answers to the [http://molviz.org/mhc/MHC-questions.pdf questions provided in a document] with the Major Histocompatibility Complex (MHC) tutorial available through [http://molviz.org MolviZ.Org]. Suggestions and feedback to [[Special:Emailuser/Eric_Martz|Eric Martz]]. |
<ol><li> | <ol><li> | ||
| - | <!--1--> | + | <!--1-->"Major Histocompatibility Complex". Early studies of tissue grafts between individuals of the same species (allografts) discovered that MHC differences caused the most rapid and intense rejection by the immune system, hence "major". Other genetic differences causes slower, less intense rejection, and were termed "minor histocompatibility" genetic loci. The normal functions of MHC were not discovered until decades later. |
| - | </li | + | </li><li> |
| - | <!--2--> | + | <!--2-->All nucleated cells express MHC class I, although the levels of expression vary from tissue to tissue (and a few cell types express undetectable levels). Red blood cells, which lack nuclei, do not express MHC class I. A virus can target any cell type. In order for cytotoxic T cells to detect virus infection, all cell types must express MHC class I. |
| - | </li | + | </li><li> |
| - | <!--3--> | + | <!--3-->Only "professional" antigen-presenting cells (APC), such as dendritic cells and macrophages, constitutively express class II MHC. B lymphocytes also express MHC class II, which enables them to receive help from T helper cells. Professional APC phagocytose or endocytose whatever they encounter. B lymphocytes endocytose the specific antigen bound by their antibody receptors, presenting the resulting peptides on their MHC class II to Th cells. |
| - | </li | + | </li><li> |
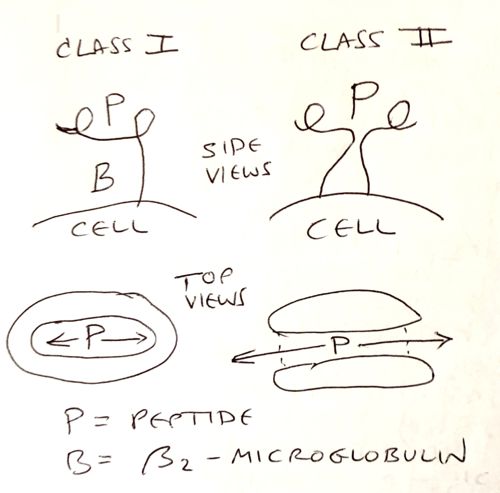
| - | <!--4--> | + | <!--4-->[[Image:Mhc-schematic-sketches.jpg|500px]] |
| - | </li | + | </li><li> |
| - | <!--5--> | + | <!--5-->T cytotoxic cells (Tc) express CD8 as a cell membrane receptor. CD8 binds to a specific binding site on MHC class I, facilitating the response of Tc to foreign peptides presented by MHC class I. |
| - | </li | + | </li><li> |
| - | <!--6--> | + | <!--6-->Protein antigens are synthesized by intracellular parasites such as viruses. Cancer cells also synthesize abnormal proteins. A portion of all cytoplasmic proteins, particularly "worn out" proteins tagged with ubiquitin, are degraded by proteasomes. Peptides generated by proteasomes are transported into the endoplasmic reticulum, and those that fit into the grooves of MHC class I molecules are then carried to the cell surface and presented by the MHC class I molecules. |
| - | + | Extracellular proteins are taken in by professional antigen-presenting cells and degraded in lysosomes. Peptides from the lysosomes bind to MHC class II, and are then presented on the cell surface. | |
| - | < | + | </li><li> |
| - | < | + | <!--7-->The peptide-binding groove of MHC class I has closed ends, and can accommodate only peptides of length 8-10 amino acids. In contrast, the groove if MHC class II has open ends, and binds peptides of 13 amino acids up to about 20 amino acids in length. |
| - | < | + | </li><li> |
| - | < | + | <!--8-->Each individual has multiple alleles of MHC. These alleles vary in the amino acid sequences in their peptide-binding grooves, thus accommodating a wide range of peptides. In addition, the alpha chains of the MHC class II alleles can assemble with multiple possible beta chains, producing additional variation in the MHC class II grooves. |
| - | < | + | </li><li> |
| - | < | + | <!--9-->Each MHC class I groove has sockets that require specific amino acid sidechains pointing down into the groove at specific positions. These are called the peptide's "anchor residues". Thus, all peptides that bind into the groove of a particular MHC class I allele have the same, or similar, anchor residues in the same positions. |
| - | < | + | </li><li> |
| - | < | + | <!--10-->MHC molecules bind peptides that fit their grooves without regard to whether those peptides are from self or foreign proteins. It is the T lymphocytes that decide. T lymphocytes let self proteins pass, but activate immune defenses when foreign peptides are detected. T lymphocytes that recognize self peptides are deleted in the thymus, so they never get out into the body. However, sometimes this T lymphocyte "education" system fails, and then auto-immune disease may occur. |
| - | < | + | </li><li> |
| - | + | <!--11-->Viruses and some cancers survive better if their foreign peptides are NOT presented to CD8 Tc cells by MHC class I. Therefore they have evolved schemes to suppress expression of MHC class I. Cells that do not express normal levels of MHC class I may be killed by Natural Killer Cells. | |
| - | + | ||
| - | <!-- | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
</li></ol> | </li></ol> | ||
Current revision
Answers to Open-Ended Questions about MHC
Here are answers to the questions provided in a document with the Major Histocompatibility Complex (MHC) tutorial available through MolviZ.Org. Suggestions and feedback to Eric Martz.
- "Major Histocompatibility Complex". Early studies of tissue grafts between individuals of the same species (allografts) discovered that MHC differences caused the most rapid and intense rejection by the immune system, hence "major". Other genetic differences causes slower, less intense rejection, and were termed "minor histocompatibility" genetic loci. The normal functions of MHC were not discovered until decades later.
-
All nucleated cells express MHC class I, although the levels of expression vary from tissue to tissue (and a few cell types express undetectable levels). Red blood cells, which lack nuclei, do not express MHC class I. A virus can target any cell type. In order for cytotoxic T cells to detect virus infection, all cell types must express MHC class I.
-
Only "professional" antigen-presenting cells (APC), such as dendritic cells and macrophages, constitutively express class II MHC. B lymphocytes also express MHC class II, which enables them to receive help from T helper cells. Professional APC phagocytose or endocytose whatever they encounter. B lymphocytes endocytose the specific antigen bound by their antibody receptors, presenting the resulting peptides on their MHC class II to Th cells.
-
T cytotoxic cells (Tc) express CD8 as a cell membrane receptor. CD8 binds to a specific binding site on MHC class I, facilitating the response of Tc to foreign peptides presented by MHC class I.
-
Protein antigens are synthesized by intracellular parasites such as viruses. Cancer cells also synthesize abnormal proteins. A portion of all cytoplasmic proteins, particularly "worn out" proteins tagged with ubiquitin, are degraded by proteasomes. Peptides generated by proteasomes are transported into the endoplasmic reticulum, and those that fit into the grooves of MHC class I molecules are then carried to the cell surface and presented by the MHC class I molecules.
Extracellular proteins are taken in by professional antigen-presenting cells and degraded in lysosomes. Peptides from the lysosomes bind to MHC class II, and are then presented on the cell surface.
-
The peptide-binding groove of MHC class I has closed ends, and can accommodate only peptides of length 8-10 amino acids. In contrast, the groove if MHC class II has open ends, and binds peptides of 13 amino acids up to about 20 amino acids in length.
-
Each individual has multiple alleles of MHC. These alleles vary in the amino acid sequences in their peptide-binding grooves, thus accommodating a wide range of peptides. In addition, the alpha chains of the MHC class II alleles can assemble with multiple possible beta chains, producing additional variation in the MHC class II grooves.
-
Each MHC class I groove has sockets that require specific amino acid sidechains pointing down into the groove at specific positions. These are called the peptide's "anchor residues". Thus, all peptides that bind into the groove of a particular MHC class I allele have the same, or similar, anchor residues in the same positions.
-
MHC molecules bind peptides that fit their grooves without regard to whether those peptides are from self or foreign proteins. It is the T lymphocytes that decide. T lymphocytes let self proteins pass, but activate immune defenses when foreign peptides are detected. T lymphocytes that recognize self peptides are deleted in the thymus, so they never get out into the body. However, sometimes this T lymphocyte "education" system fails, and then auto-immune disease may occur.
-
Viruses and some cancers survive better if their foreign peptides are NOT presented to CD8 Tc cells by MHC class I. Therefore they have evolved schemes to suppress expression of MHC class I. Cells that do not express normal levels of MHC class I may be killed by Natural Killer Cells.