We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Protegrin
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 3: | Line 3: | ||
__Toc__ | __Toc__ | ||
==About this Structure== | ==About this Structure== | ||
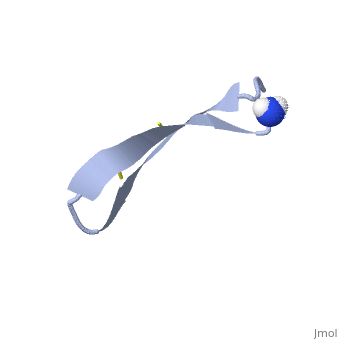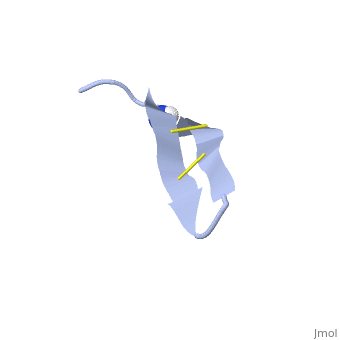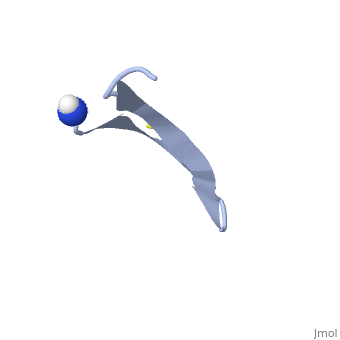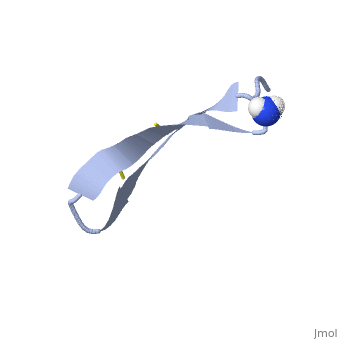
| - | 1PG1 is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Sus_scrofa Sus scrofa]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PG1 OCA]. 1PG1 is an arginine and cysteine rich protein, which forms a double stranded anti-parallel β-sheet structure. The single chain forms a membrane-bound dimer, of structure [http://proteopedia.org/wiki/index.php/1zy6 1ZY6]. The structure of 1PG1 is similar to that of some other [http://en.wikipedia.org/wiki/Antimicrobial_peptide antimicrobial peptides] such as [http://en.wikipedia.org/wiki/Defensin defensins]<ref>Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/2668334 2668334]</ref>. In one study comparing the susceptibility of [http://en.wikipedia.org/wiki/Chlamydia_trachomatis Chlamydia trachomatis] to 1PG1 and a similar defensin peptide, 1PG1 was shown to be significantly more effective at inactivating the bacteria<ref>Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/8641770 8641770]</ref>. The antimicrobial action of this protein is believed to be due to its ability to create pores in bacterial membranes causing ion leakage <ref>Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10446287 10446287]</ref>. This antimicrobial activity has given rise to the idea of using the peptide as a therapeutic for local or systemic infections<ref>Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163996/ PMC163996]</ref>. The protegrin is synthesized as a ca. 149 amino acid precursor with a cathelin-like domain. | + | '''Protegrin 1''' (1PG1) is a [[Single protein]] structure of sequence from [http://en.wikipedia.org/wiki/Sus_scrofa Sus scrofa]. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=1PG1 OCA]. 1PG1 is an arginine and cysteine rich protein, which forms a double stranded anti-parallel β-sheet structure. The single chain forms a membrane-bound dimer, of structure [http://proteopedia.org/wiki/index.php/1zy6 1ZY6]. The structure of 1PG1 is similar to that of some other [http://en.wikipedia.org/wiki/Antimicrobial_peptide antimicrobial peptides] such as [http://en.wikipedia.org/wiki/Defensin defensins]<ref>Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/2668334 2668334]</ref>. In one study comparing the susceptibility of [http://en.wikipedia.org/wiki/Chlamydia_trachomatis Chlamydia trachomatis] to 1PG1 and a similar defensin peptide, 1PG1 was shown to be significantly more effective at inactivating the bacteria<ref>Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: [http://www.ncbi.nlm.nih.gov/pubmed/8641770 8641770]</ref>. The antimicrobial action of this protein is believed to be due to its ability to create pores in bacterial membranes causing ion leakage <ref>Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/10446287 10446287]</ref>. This antimicrobial activity has given rise to the idea of using the peptide as a therapeutic for local or systemic infections<ref>Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC163996/ PMC163996]</ref>. The protegrin is synthesized as a ca. 149 amino acid precursor with a cathelin-like domain. |
==Gene Ontology<ref>NPG1. EBI QuickGO. [http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194 http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194]</ref>== | ==Gene Ontology<ref>NPG1. EBI QuickGO. [http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194 http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194]</ref>== | ||
| Line 16: | Line 16: | ||
*'''Superfamily:''' [http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.html Antimicrobial beta-hairpin] | *'''Superfamily:''' [http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.html Antimicrobial beta-hairpin] | ||
*'''Family:''' [http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.f.html theta defensin-like] | *'''Family:''' [http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.f.html theta defensin-like] | ||
| - | </ | + | |
| + | ==3D structures of protegrin== | ||
| + | |||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | |||
| + | [[1pg1]], [[1zy6]] – pPRO1 – pig – NMR<BR /> | ||
| + | [[2muh]] - pPRO2 residues 131-146 - NMR<br /> | ||
| + | [[1n5h]], [[1n5p]] – pPRO3 cathelin-like domain – NMR<br /> | ||
| + | [[1kwi]], [[1lxe]], [[1pfp]] – pPRO3 cathelin-like domain <br /> | ||
| + | [[2m2z]], [[2mz6]] - pPRO3 residues 131-148 - NMR<br /> | ||
| + | [[6qkf]] – pPRO4 residues 131-148 - NMR <br /> | ||
| + | [[2nc7]] – pPRO5 residues 131-148 - NMR <br /> | ||
| + | |||
==References== | ==References== | ||
Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes., Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J, Chem Biol. 1996 Jul;3(7):543-50. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8807886 8807886] | Solution structure of protegrin-1, a broad-spectrum antimicrobial peptide from porcine leukocytes., Fahrner RL, Dieckmann T, Harwig SS, Lehrer RI, Eisenberg D, Feigon J, Chem Biol. 1996 Jul;3(7):543-50. PMID:[http://www.ncbi.nlm.nih.gov/pubmed/8807886 8807886] | ||
| - | + | </StructureSection> | |
[[Category: Single protein]] | [[Category: Single protein]] | ||
[[Category: Sus scrofa]] | [[Category: Sus scrofa]] | ||
| Line 30: | Line 42: | ||
[[Category: Antimicrobial peptide]] | [[Category: Antimicrobial peptide]] | ||
| - | ==3D structures of protegrin== | ||
| - | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | |||
| - | [[1pg1]], [[1zy6]] – pPRO1 – pig – NMR<BR /> | ||
| - | [[2muh]] - pPRO2 residues 131-146 - NMR<br /> | ||
| - | [[1n5h]], [[1n5p]] – pPRO3 cathelin-like domain – NMR<br /> | ||
| - | [[1kwi]], [[1lxe]], [[1pfp]] – pPRO3 cathelin-like domain <br /> | ||
| - | [[2m2z]] - pPRO3 residues 131-148 - NMR<br /> | ||
| - | [[6qkf]] – pPtn-4 - NMR <br /> | ||
{{Reflist}} | {{Reflist}} | ||
Current revision
| |||||||||||
- ↑ Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity., Lehrer RI, Barton A, Daher KA, Harwig SS, Ganz T, Selsted ME., J Clin Invest. 1989 Aug;84(2):553-61. PMID: 2668334
- ↑ Susceptibility of Chlamydia trachomatis to protegrins and defensins., Yasin B, Harwig SS, Lehrer RI, Wagar EA., Infect Immun. 1996 Mar;64(3):709-13. PMID: 8641770
- ↑ Membrane channel formation by antimicrobial protegrins., Sokolov Y, Mirzabekov T, Martin DW, Lehrer RI, Kagan BL, Biochim Biophys Acta. 1999 Aug 20;1420(1-2):23-9. PMID:10446287
- ↑ Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity, D A Steinberg, M A Hurst, C A Fujii, A H Kung, J F Ho, F C Cheng, D J Loury, and J C Fiddes, Antimicrob Agents Chemother. 1997 August; 41(8): 1738–1742. PMCID: PMC163996
- ↑ NPG1. EBI QuickGO. http://www.ebi.ac.uk/QuickGO/GProtein?ac=P32194
- ↑ Protein: Protegrin 1 (PG1) from Pig (Sus scrofa). Structural Classification of Proteins. http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.ba.e.b.f.b.html
Created with the participation of Lee Tien.