SN1 reaction
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
== S<sub>N</sub>1-Substitution of Cl<sup>-</sup> and ''tert''-Butanol == | == S<sub>N</sub>1-Substitution of Cl<sup>-</sup> and ''tert''-Butanol == | ||
| - | <StructureSection load='' size='340' side='right' caption='' scene='54/542276/Side_view/ | + | <StructureSection load='' size='340' side='right' caption='' scene='54/542276/Side_view/3'> |
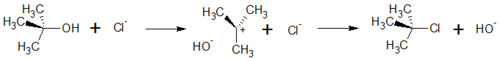
The S<sub>N</sub>1 reaction belongs to the basic reaction in organic chemistry. The number 1 says that it is a monomolecular reaction. This means that in the rate determining step of the reaction, only one of the educts is involved. The kinetic of the reaction therefore follows the reation rate of first order. | The S<sub>N</sub>1 reaction belongs to the basic reaction in organic chemistry. The number 1 says that it is a monomolecular reaction. This means that in the rate determining step of the reaction, only one of the educts is involved. The kinetic of the reaction therefore follows the reation rate of first order. | ||
| - | In general, substitutions exchange substituents in an organic molecule. One example of an S<sub>N</sub>1 | + | In general, substitutions exchange substituents in an organic molecule. One example of an S<sub>N</sub>1 reaction is the exchange of the Hydroxide in <i>tert</i>-Butanol by a Chloride Ion or |
In general, SN1 substitution can take place when a stable carbocation can be formed. If not, the reaction follows the SN2 mechanism. | In general, SN1 substitution can take place when a stable carbocation can be formed. If not, the reaction follows the SN2 mechanism. | ||
| Line 23: | Line 23: | ||
<jmolButton><script>if(_animating);anim off;else;frame play;endif</script><text>Toggle animation</text></jmolButton> | <jmolButton><script>if(_animating);anim off;else;frame play;endif</script><text>Toggle animation</text></jmolButton> | ||
</jmol> | </jmol> | ||
| + | <jmol> | ||
| + | <jmolButton><script>spacefill 90; dots on; wireframe 30;</script><text>translucent atoms</text></jmolButton> | ||
| + | <jmolButton><script>spacefill on; dots off;</script><text>spacefilling atoms</text></jmolButton> | ||
| + | <jmolButton><script>spacefill 90; dots off; wireframe 30;</script><text>backbone</text></jmolButton> | ||
| + | </jmol> | ||
| + | |||
===See also=== | ===See also=== | ||
| - | [[SN2_reaction|S<sub>N</sub>2 reaction: Substitution of chloride and methanol]] | + | [[SN2_reaction|S<sub>N</sub>2 reaction: Substitution of chloride and methanol]]<br> |
| + | [[Esterification|Esterification]]<br> | ||
| + | [[Ozonolysis|Ozonolysis - a type of cycloaddition]] | ||
== References == | == References == | ||
This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn1_substutition/sn1_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project | This demo was adapted from http://www.chemieunterricht-interaktiv.de/en/animations/sn1_substutition/sn1_substitution_3d.html by Dr. V. Pietzner, part of the ChiLe project | ||
<references/> | <references/> | ||
</StructureSection> | </StructureSection> | ||
| + | [[Category: BioMolViz]] | ||
| + | [[Category: Chemical Reactions]] | ||
Current revision
SN1-Substitution of Cl- and tert-Butanol
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Joel L. Sussman, Jaime Prilusky, Angel Herraez, Verena Pietzner