Sandbox Reserved 1628
From Proteopedia
(Difference between revisions)
| (15 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_Reserved_BHall_F20}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_Reserved_BHall_F20}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | == | + | ==Epithelial Adhesin 1A== |
| - | <StructureSection load='4af9' size='340' side='right' caption=' | + | <StructureSection load='4af9' size='340' side='right' caption='EpaA in a complex with Galb1-3Glc' scene=''> |
| - | + | Adhesins, commonly found on the surface of infections organisms, aid in binding or adhering the pathogen to other cells. This is normally the beginning of an infection as the pathogen can bind to a host cell and potentially start a cascade of events within the host cell that leads to an infection<ref>PMID:9973330</ref>. Various pathogens have various types of adhesin proteins, but this page looks specifically at epithelial adhesins, particularly one found in ''Candida glabrata'', a fungi which causes infections most often in immunosuppressed patients<ref name="yeast">PMID:9880475</ref> and can lead to deadly bloodstream infections. | |
| - | + | ||
== Function of your Protein == | == Function of your Protein == | ||
| - | + | ''Candida glabrata'' is a fungal organism that commonly interacts with other similar fungi such as ''Candida albicans'' to infect humans with what are commonly known as thrush, yeast infections, and urinary tract infections<ref name="yeast" />. The fungi is a type of yeast which grows on the human body naturally, it's only once it enters the body somehow that it starts to cause infections. For the pathogen to do this, it must first adhere to a host cell and does so by using the adhesins found on the outside of the fungi. | |
| + | |||
| + | EpaA1 looks specifically for certain sugars on the outside of the host cells and will bind to those. <scene name='86/861610/Ligandhighlight/2'>This specific adhesin</scene> binds to beta-D-galactopyranose-(1,3)-beta-D-glucopyranose. For ''Candida glabrata'', there are about 20 different types of epithelial adhesins, each with conserved areas of the binding site and variable areas to be able to bind to a variety of sugars on the host cell<ref name="journal">PMID:32669365</ref>. | ||
== Biological relevance and broader implications == | == Biological relevance and broader implications == | ||
| + | |||
| + | For ''Candida glabrata'' to infect host cells, it must first attach itself to said cell. By having the active site within the protein have both conserved and variable areas, it allows the fungal pathogen to have a wider array of hosts it can bind to. This also leads to it being resistant for various treatments commonly used for similar fungi and can potentially enter and infect the bloodstream of a patient. | ||
| + | |||
| + | Current research is investigating which sugars the binding site works best with, what parts of the binding site they interact with, and how potential treatments can be made from these findings. By determining which pieces of the binding site are conserved, new drugs can be produced that can interact with those areas and prevent the adhesin from binding to an actual host cell. This is especially necessary for those who are immunocompromised and may be at a higher risk of not only becoming infected with the fungal pathogen, but dying from the resulting bloodstream infection. | ||
== Important amino acids == | == Important amino acids == | ||
| + | |||
| + | The ligand for this specific EpaA1 is the sugar <scene name='86/861610/Bdglacbdglc/3'>beta-D-galactopyranose-(1,3)-beta-D-glucopyranose</scene>. To keep the sugar bound, within the active site there are 4 amino acids which hydrogen bind to it - <scene name='86/861610/Activesiteaa/2'>Arg226, Asp164, Asp165, and Glu227</scene>. The Arg and Asp amino acids are very conserved between the various epithelial adhesin active sites and are considered critical for effective binding whereas the Glu is more variable and specific to binding this sugar. | ||
== Structural highlights == | == Structural highlights == | ||
| + | |||
| + | The majority of the <scene name='86/861610/Secondarystructure/1'>protein's secondary structure</scene> is comprised of antiparallel beta sheets with loops connecting them. Two loops help to form a calcium binding pocket which helps to bind the calcium ion shown and three other loops form the outer pocket. These loops are highly variable in their composition and are contributed with recognizing certain sugars that the inner binding site prefer to bind to. | ||
| + | |||
| + | The tertiary structure allows the loops created by the antiparallel beta sheets <scene name='86/861610/Tertiarystructure/1'>to come close enough to interact with one another</scene>. These loops help to stabilize the calcium ion shown in blue as well as form the outer pocket. Between two of the variable loops is a disulfide bond created by two cystine amino acids which is the only conserved area within the loops. The disulfide bond helps to maintain structure of the outer pocket and is consistent between a variety of the epithelial adhesins. | ||
| + | |||
| + | The protein itself has <scene name='86/861610/Spacefill/2'>a small indent near the active site</scene> where the calcium ion sits just sticking out to interact with sugar ligands. The cleft into the protein fits a very specific size of sugar, which follows along with the current research that shows only a few different pyranose sugars can interact with the adhesins<ref name="journal" />. | ||
== Other important features == | == Other important features == | ||
| - | + | Although not bound to the ligand, <scene name='86/861610/Tryptophan/1'>tryptophan interacts with it</scene> through hydrophobic interactions and maintains its position within the active site. Changing the amino acid to one without an aromatic ring decreased the affinity for the ligand to bind to the adhesin, while switching it with an amino acid that also has an aromatic ring resulted in no overall change. This shows the most important feature of this specific amino acid - the aromatic ring interacts with the ligand by creating a hydrophobic interaction to stabilize it. | |
| + | |||
| + | The calcium ion featured within the active site is not a ligand itself as it's necessary for the protein to work and bind to the sugar ligand. Most of the bonds formed from the ligand are connected to the calcium ion in metal coordination complexes, which in turn is bound to the protein. Two conserved loops within multiple paralogs of the protein are specifically to make sure the calcium ion is within the correct position. | ||
| + | |||
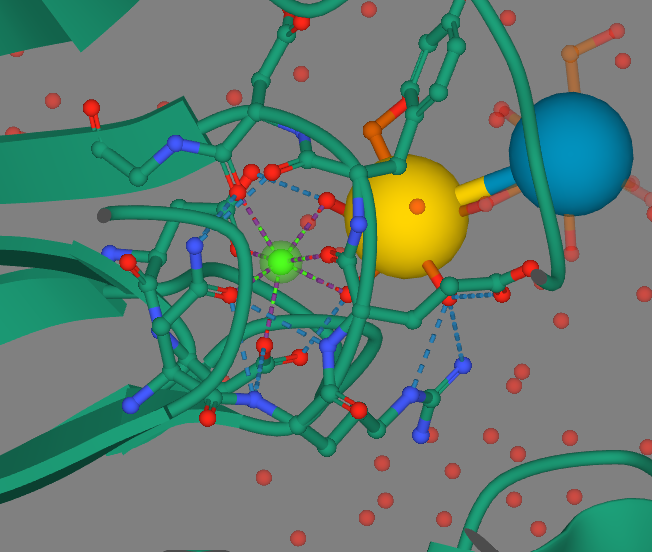
| + | [[Image:CalciumComplex.png]] | ||
| + | |||
| + | Calcium is in green, the yellow and blue spheres represent the sugar ligand, the purple branches out from calcium are metal coordination complexes, and the blue dashed branches are hydrogen bonds. | ||
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
| + | |||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from 09/18/2020 through 03/20/2021 for use in CHEM 351 Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, IA. This reservation includes Sandbox Reserved 1628 through Sandbox Reserved 1642. |
To get started:
More help: Help:Editing |
Epithelial Adhesin 1A
| |||||||||||
References
- ↑ Soto GE, Hultgren SJ. Bacterial adhesins: common themes and variations in architecture and assembly. J Bacteriol. 1999 Feb;181(4):1059-71. doi: 10.1128/JB.181.4.1059-1071.1999. PMID:9973330 doi:http://dx.doi.org/10.1128/JB.181.4.1059-1071.1999
- ↑ 2.0 2.1 Fidel PL Jr, Vazquez JA, Sobel JD. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev. 1999 Jan;12(1):80-96. PMID:9880475
- ↑ 3.0 3.1 Hoffmann D, Diderrich R, Reithofer V, Friederichs S, Kock M, Essen LO, Mosch HU. Functional reprogramming of Candida glabrata epithelial adhesins: the role of conserved and variable structural motifs in ligand binding. J Biol Chem. 2020 Jul 15. pii: RA120.013968. doi: 10.1074/jbc.RA120.013968. PMID:32669365 doi:http://dx.doi.org/10.1074/jbc.RA120.013968