User:Lizun Xin/Mtb BlaC Inhibition
From Proteopedia
< User:Lizun Xin(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | Drug resistant strain of ''Mycobacterium tuberculosis (Mtb)'' propose a major medical problem today, as traditional β-lactam antibiotics does not exhibited effective treatment. From previous study we understand that the resistance was due to the expression of <scene name='87/871911/Asymmetric_unit_of_blac/ | + | Drug resistant strain of ''Mycobacterium tuberculosis (Mtb)'' propose a major medical problem today, as traditional β-lactam antibiotics does not exhibited effective treatment. From previous study we understand that the resistance was due to the expression of <scene name='87/871911/Asymmetric_unit_of_blac/4'>BlaC</scene> protein in ''Mtb''. <StructureSection load='3ZHH' size='340' side='right' caption='Caption for this structure' scene=''> |
==Background on The Disease == | ==Background on The Disease == | ||
Tuberculosis (TB) is a human respiratory disease, which is caused by bacteria: Mycobacterium tuberculosis (Mtb). The most common pathway of transmission for M. tuberculosis is by airborne droplets, for example, coughing and sneezing. M. bovis can be transmitted by animal products, for example uncooked meat and unpasteurised milk. The bacterium can hibernate in the human body for various times, from week to years. TB usually affects the lungs, but in some cases, the infection can spread outside of the lungs (eg. lymphatic system) <sup>[1]</sup>. | Tuberculosis (TB) is a human respiratory disease, which is caused by bacteria: Mycobacterium tuberculosis (Mtb). The most common pathway of transmission for M. tuberculosis is by airborne droplets, for example, coughing and sneezing. M. bovis can be transmitted by animal products, for example uncooked meat and unpasteurised milk. The bacterium can hibernate in the human body for various times, from week to years. TB usually affects the lungs, but in some cases, the infection can spread outside of the lungs (eg. lymphatic system) <sup>[1]</sup>. | ||
| Line 25: | Line 25: | ||
Initiation: First, the amine group of Lys-73 deprotonates Ser-70 with its lone pair of electrons. Then the reaction starts with an esterification step; the lone pair on oxygen of Ser-70 attacks the β-lactam carbon forming a covalent bond. Meanwhile, the C-N bond in beta-lactam ring is hydrolysed. Hence the βbeta-lactam is destroyed. | Initiation: First, the amine group of Lys-73 deprotonates Ser-70 with its lone pair of electrons. Then the reaction starts with an esterification step; the lone pair on oxygen of Ser-70 attacks the β-lactam carbon forming a covalent bond. Meanwhile, the C-N bond in beta-lactam ring is hydrolysed. Hence the βbeta-lactam is destroyed. | ||
| - | In this view | + | In <scene name='87/871911/3n7w_amoxicillin_bounded/2'>this view</scene>, amoxicillin is covalently bounded to BlaC in the active site. |
Re-activating BlaC: the second step of the mechanism involves the hydrolysis of ester linkage with Lys-73, which allows the BlaC enzyme be reactivated. | Re-activating BlaC: the second step of the mechanism involves the hydrolysis of ester linkage with Lys-73, which allows the BlaC enzyme be reactivated. | ||
| Line 44: | Line 44: | ||
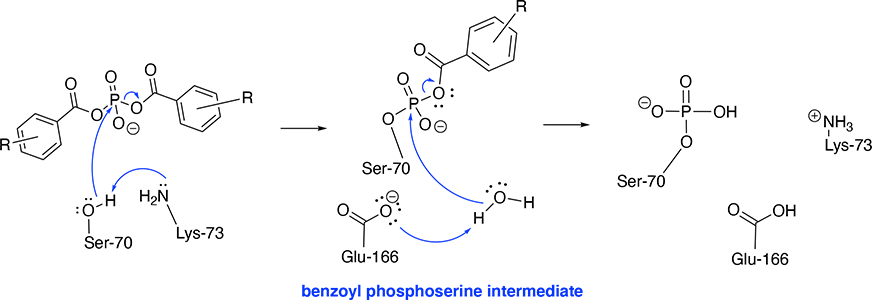
Initiation: The amine side chain of Lys-73 deprotonates the Ser-70 hydroxy group, which instantaneously attacks the phosphorus atom resulting in the cleavage of one benzoate group. <sup>[3]</sup> | Initiation: The amine side chain of Lys-73 deprotonates the Ser-70 hydroxy group, which instantaneously attacks the phosphorus atom resulting in the cleavage of one benzoate group. <sup>[3]</sup> | ||
| - | Final Step: Glu-166 deprotonates a water molecule which cleaves the remaining benzoate group. Hence, the Ser-70 is phosphorylated | + | Final Step: Glu-166 deprotonates a water molecule which cleaves the remaining benzoate group. Hence, the <scene name='87/871911/6n14_phosphorylated/1'>Ser-70 is phosphorylated</scene>. |
== Treatments == | == Treatments == | ||
Current revision
Drug resistant strain of Mycobacterium tuberculosis (Mtb) propose a major medical problem today, as traditional β-lactam antibiotics does not exhibited effective treatment. From previous study we understand that the resistance was due to the expression of protein in Mtb.
| |||||||||||