User:Justin Smith/Sandbox 1
From Proteopedia
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==DGAT Homo Sapien== | ==DGAT Homo Sapien== | ||
| - | <StructureSection load=' | + | <StructureSection load='6VP0' size='340' side='right' caption='Caption for this structure' scene=''> |
== Function == | == Function == | ||
The triacylglycerol biosynthesis pathway was elucidated nearly 60 years ago | The triacylglycerol biosynthesis pathway was elucidated nearly 60 years ago | ||
| Line 8: | Line 8: | ||
DGAT enzymes were identified more than 20 years ago | DGAT enzymes were identified more than 20 years ago | ||
| - | Polytopic Endoplasmic Reticulum Membrane Protein | + | Polytopic Endoplasmic Reticulum [https://en.wikipedia.org/wiki/Membrane_protein Membrane Protein] |
Embedded in membrane of ER | Embedded in membrane of ER | ||
| Line 17: | Line 17: | ||
== Disease == | == Disease == | ||
| + | ==Active Site== | ||
| + | <scene name='87/877628/His415/1'>His415 </scene>is the active base catalytic function | ||
== Relevance == | == Relevance == | ||
| + | |||
| + | ==Mecanism== | ||
| + | [[Image:Mec.PNG|300 px|left|thumb|Figure 1. Mecanism of base catalyized reaction]] | ||
== Structural highlights == | == Structural highlights == | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | + | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.<ref name="wang">PMID:32433610</ref>. |
| + | |||
| + | ==Tunnels== | ||
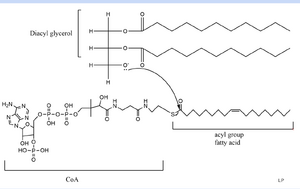
| + | DGAT consists of 3 tunnels, a cytosolic tunnel, an ER-luminal funnel, and a membrane-embedded lateral gate. The cytosolic tunnel is the site of acyl-CoA binding, with the CoA group pointing at the cytosolic face and its acyl chain pointing towards the endoplasmic reticulum lumen. DAG then enters via the lateral gate on the luminal side via the lateral gate where it can then access the active site. The resulting product can then be released to either side of the membrane. <ref name="Sui">PMID:32433611</ref>. | ||
| - | </StructureSection> | ||
== References == | == References == | ||
| + | |||
| + | |||
| + | |||
| + | |||
<references/> | <references/> | ||
==Student Contributers== | ==Student Contributers== | ||
*Justin Smith | *Justin Smith | ||
Current revision
Contents |
DGAT Homo Sapien
| |||||||||||
Disease
Active Site
is the active base catalytic function
Relevance
Mecanism
Structural highlights
This is a sample scene created with SAT to by Group, and another to make of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes.[1].
Tunnels
DGAT consists of 3 tunnels, a cytosolic tunnel, an ER-luminal funnel, and a membrane-embedded lateral gate. The cytosolic tunnel is the site of acyl-CoA binding, with the CoA group pointing at the cytosolic face and its acyl chain pointing towards the endoplasmic reticulum lumen. DAG then enters via the lateral gate on the luminal side via the lateral gate where it can then access the active site. The resulting product can then be released to either side of the membrane. [2].
References
- ↑ Wang L, Qian H, Nian Y, Han Y, Ren Z, Zhang H, Hu L, Prasad BVV, Laganowsky A, Yan N, Zhou M. Structure and mechanism of human diacylglycerol O-acyltransferase 1. Nature. 2020 May;581(7808):329-332. doi: 10.1038/s41586-020-2280-2. Epub 2020 May, 13. PMID:32433610 doi:http://dx.doi.org/10.1038/s41586-020-2280-2
- ↑ Sui X, Wang K, Gluchowski NL, Elliott SD, Liao M, Walther TC, Farese RV Jr. Structure and catalytic mechanism of a human triacylglycerol-synthesis enzyme. Nature. 2020 May;581(7808):323-328. doi: 10.1038/s41586-020-2289-6. Epub 2020 May, 13. PMID:32433611 doi:http://dx.doi.org/10.1038/s41586-020-2289-6
Student Contributers
- Justin Smith