User:Haylie Moehlenkamp/Sandbox1
From Proteopedia
< User:Haylie Moehlenkamp(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | =Acyl-Coenzyme Cholesterol Acetyltransferase ( | + | =Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1)= |
| - | <StructureSection load='6p2p' size='340' side='right' caption='ACAT' scene | + | <StructureSection load='6p2p' size='340' side='right' caption='ACAT' scene='87/877605/6p2p_dimer/2'> |
| - | + | ||
==Introduction== | ==Introduction== | ||
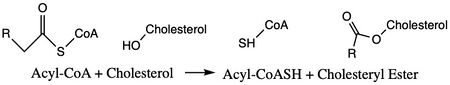
| - | Acyl-Coenzyme A Cholesterol Acyltransferase ( | + | Acyl-Coenzyme: A Cholesterol Acyltransferase 1 (ACAT1), or also known as Sterol ''O''-Acyltransferase (SOAT), is an important enzyme in the body. [https://en.wikipedia.org/wiki/Thiolase ACAT] is an important enzyme that catalyzes the esterification of cholesterol to form cholesterol esters, and it belongs to the class of enzymes called acyltransferases (Figure 1). As a member of the [https://en.wikipedia.org/wiki/MBOAT MBOAT] family, it is a key enzyme in lipid metabolism. This enzyme is biologically important because it affects the solubility of cholesterol in the cell membrane and promotes accumulation of cholesterol ester in the cytoplasm as fat droplets. Accumulation of cholesterol ester as these lipid droplets is a main characteristic of macrophage foaming, which can lead to atherosclerotic diseases <ref name=”Qian”>PMID:32433614</ref>. |
| - | Cholesterol esters were found in arterial lesions in 1910, but the first ACAT activity was discovered in the mid 1900's. This led to the inhibition of ACAT as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT inhibitors grew, but some of the compounds | + | Cholesterol esters were found in arterial lesions in 1910, but the first ACAT activity was discovered in the mid 1900's. This led to the inhibition of ACAT as being looked at as a possible strategy of preventing or treating atherosclerosis. Between 1980-1995, the interest in ACAT inhibitors grew, but some of the compounds developed exhibited toxicity. As they were looking into the function of the ACAT1 gene, ACAT2 was discovered. In 1993, an ACAT gene was successfully cloned. This discovery led to more studies with ACAT and atherosclerosis. Some of these studies used mice and also showed cellular toxicity. ACAT inhibition is still being pursued as a strategy for treatment or prevention of atherosclerosis and related diseases. |
<ref name=”Farese Jr.”>PMID: 16857957</ref> | <ref name=”Farese Jr.”>PMID: 16857957</ref> | ||
| - | [[Image: | + | [[Image:simplemechanismforACAT.jpg|450 px|left|thumb|Figure 1. Chemical Structures for the Reactants and Products of ACAT1]] |
| - | + | ||
==Structural Overview== | ==Structural Overview== | ||
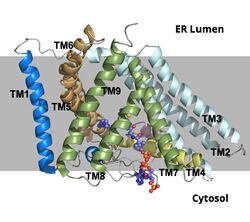
| - | + | ACAT1 was experimentally determined as a [https://en.wikipedia.org/wiki/Tetramer tetramer] of size approximately 260 kDa, composed of helices and loops. Each monomer of the tetramer contains 9 transmembrane helices (Figure 3). However, the dimer of ACAT1 was found to be the biologically active arrangement. | |
| - | + | ||
| - | + | ||
| - | === | + | This <scene name='87/877605/Tetramer/1'>tetramer</scene> is about 260 kDa and is composed of helices and loops, with each monomer containing 9 transmembrane helices (Figure 3). The <scene name='87/877604/Colored_dimer/4'>dimer of ACAT</scene> was found to be the active arrangement. |
| - | + | [[Image:Screen_Shot_2021-04-20_at_3.29.54_PM.jpg|400 px|left|thumb|Figure 2. ACAT1 Dimer in the Membrane. The gray shaded region is the plane of the bilipid membrane. The tunnels where molecules enter and exit are labeled. The ER lumen is at the top with the cytosol at the bottom.]] | |
| + | ===Dimer-Dimer Interface=== | ||
| + | The <scene name='87/877604/Dimer_interface/17'>dimer-dimer interface</scene> is mobile and mostly hydrophobic, and the residues interact in a shape-complementary manner. It was also found that the reaction chamber is shielded by a lid from the cytosolic side, which leads to low catalytic activity. The binding of acyl-CoA and cholesterol induce conformational changes that activate the tunnels necessary for substrates to enter them. Work is still being done to fully determine the mechanism of this reaction, but this is the proposed pathway. | ||
| + | [[Image:Screen_Shot_2021-04-20_at_3.30.39_PM.jpg|250 px|right|thumb|Figure 3. ACAT1 Monomer in the Membrane. This shows the 9 transmembrane helices. Each helix is labeled and colored according to the different domains. The ligand is shown as ball and stick.]] | ||
===Tunnels=== | ===Tunnels=== | ||
| - | The catalytic site is accessed through three different tunnels that lead from the center catalytic | + | The catalytic site is accessed through three different tunnels that lead from the center catalytic chamber of the monomer, to the [http://en.wikipedia.org/wiki/Lumen_(anatomy) lumen], cytosol, and transmembrane spaces. The tunnels allow the introduction of reactants into the acyl transferase mechanism and the exit of the products to the correct location depending on their function. The cholesterol enters through the T tunnel while the acyl-CoA enters through the C tunnel. |
| - | The <scene name='87/877605/C_tunnel/1'>C tunnel</scene> is open to the cytosolic side of the protein in which the Acyl | + | The <scene name='87/877605/C_tunnel/1'>C tunnel</scene> is open to the cytosolic side of the protein in which the Acyl Co-A enters into the catalytic chamber. |
| - | The <scene name='87/877605/T_tunnel/1'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic | + | The <scene name='87/877605/T_tunnel/1'>T tunnel</scene> is the transmembrane tunnel in which the cholesterol enters into the catalytic region. Important <scene name='87/877605/T_tunnel_residues/1'>T tunnel residues</scene> include Arg262, Phe263, and Leu306. These residues are important for the proper entrance and orientation of the cholesterol to allow for its deprotonation in the mechanism. Upon mutation of these residues, the tunnel function was inhibited. The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> provides a potential opening to the lumen side. The enzymatic reaction occurs at the intersection of these two tunnels. It is catalyzed at the intersection of the two tunnels, where the His460 residue is located. The CoASH is released to the cytosol by way of the C tunnel, but the cholesterol ester either exits from the T tunnel to the membrane or through the L tunnel to the lumen. The specific mechanism by which the cholesterol ester product exits the tunnel to enter the lumen has not yet been determined. |
| - | The <scene name='87/877605/L_tunnel/1'>L tunnel</scene> | + | |
==Active Site== | ==Active Site== | ||
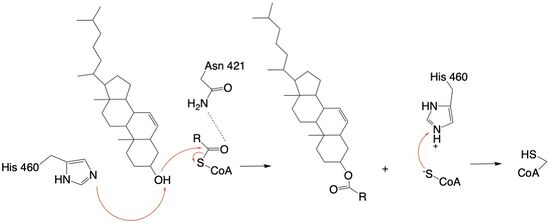
| - | The | + | The active site contains <scene name='87/877605/Catalytic_residues/1'> catalytic residues</scene> that are essential to the ACAT1 mechanism. These residues include His460 to function as a base catalyst and Asn421 which functions as transition state stabilization with hydrogen bonding. Also important for orientation of the Acyl Co-A ligand is the presence of hydrophobic residues (Trp407,Trp420) to stabilize the fatty acid. The active site is at the intersection of all three tunnels to allow a central position for the acyltransferase reaction to occur. |
| + | The His460 is positioned to deprotonate the cholesterol upon entrance into the T tunnel: Acyl Co-A upon entering is positioned to where the sulfur bonded to the carboxyl carbon is at the direct intersection of the T tunnel into the active site. | ||
==Mechanism== | ==Mechanism== | ||
| - | The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site | + | The mechanism of the [[http://en.wikipedia.org/wiki/Acyltransferase#:~:text=Acyltransferase%20is%20a%20type%20of,%2Dalcohol%20O%2Dfatty%2Dacyltransferase acyltransferace]]reaction occurs in the catalytic site of each monomer of the dimer of ACAT1. The T and and C tunnels converge to the same space to allow the proper orientation of the Acyl Co-A and the incoming cholesterol from the transmembrane. The Acyl Co-A is oriented in a way to allow the His460 to act as a base catalyst to begin the reaction by deprotonation of the cholesterol, which allows it to attack the carbonyl carbon which breaks the sulfur carbonyl bond (Figure 4). The Acyl CoA transition state is held in place by the <scene name='87/877605/Catalytic_residues/4'>oxyanion hole</scene> of Asn421. This mechanism produces Acyl-CoASH and cholesterol ester. The Acyl-CoASH leaves through the C tunnel to the cytosol. |
| - | [[Image: | + | [[Image:concise mechanism.jpg|550px|left|thumb|Figure 4: Acyltransferase mechanism of ACAT1 with conserved MBOAT family catalytic residues.]] |
| + | |||
==Inhibitor== | ==Inhibitor== | ||
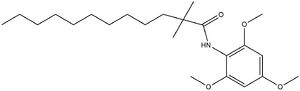
| - | <scene name='87/877626/Inhibitor/ | + | <scene name='87/877626/Inhibitor/7'>CI-976</scene> is known as a small molecule inhibitor that is part of what is called the fatty acyl amide analog family, and functions as a competitive inhibitor of Acyl Co-A <ref name "Shengcheng"> PMID:32424158</ref><ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref>. Guan discussed that this inhibitor in previous studies had shown that CI-976 reduced the size of atherosclerotic plaques and cholesterol levels in plasma <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref> . |
| - | + | The <scene name='87/877626/Overlay/13'>overlay</scene> illustrates how CI-976 can act as a competitive inhibitor of Acyl Co-A. Structurally, Acyl Co-A and CI-976 are both largely hydrophobic, each with long hydrophobic tails. As evident in this image, the hydrophobic tail of CI-976, mimics that of Acyl Co-A. This allows for the CI-976 inhibitor to be recognized by ACAT1 and to bind tightly in the active site pocket, blocking Acyl Co-A from binding, thus rendering ACAT1 unable to perform its reaction. [[Image: CI-976_chemdraw.jpg|300 px|right|thumb|Figure 5. CI-976 Inhibitor]] | |
| - | The <scene name='87/877626/Overlay/ | + | |
| - | ==Disease | + | ==Diseases== |
| + | ===Neurodegenerative Diseases and Cancers=== | ||
| + | ACAT is involved in diseases such as [https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Alzheimer's Disease] and [https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055 Parkinson’s Disease] and other neurodegenerative diseases due to the accumulation of Aβ plaques in the brain. After research on [https://www.mayoclinic.org/diseases-conditions/glioma/symptoms-causes/syc-20350251 glioma], [https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 prostate cancer], [https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 pancreatic cancer], [https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 leukemia], and [https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470 breast cancer], it has been noted that ACAT plays a role in the progression of cancer over time. Recently, Ayyagari et al. found that there was a significant increase in ACAT1 expression in ovarian cancer cell lines <ref name="Ayyagari"> doi:10.1371/journal.pone.0228024</ref>. ACAT-2 is believed to be upregulated in Nephrotic Syndrome which can lead to cardiovascular disease and renal diseases <ref name="Vaziri"> doi:10.1161/01.CIR.0000136023.70841.0F</ref>. Because of ACAT's activity in tissues such as the aorta, intestine, and liver, it plays a role in atherosclerosis. Studies have shown that the inhibition of ACAT2 can slow the progression of Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Guan discussed a previous study which found that CI-976 decreased the size of atherosclerosis plaques and the overall concentration of cholesterol in the blood plasma of animals that had been fed a high cholesterol diet <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref> | ||
| + | |||
===Alzheimer's Disease=== | ===Alzheimer's Disease=== | ||
| - | [https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Alzheimer's Disease]is a neurodegenerative disease characterized by accumulation of extracellular plaques that cause interferences with memory retrieval. These plaques are made up of | + | [https://www.mayoclinic.org/diseases-conditions/alzheimers-disease/symptoms-causes/syc-20350447 Alzheimer's Disease]is a neurodegenerative disease characterized by accumulation of extracellular plaques that cause interferences with memory retrieval. These plaques are made up of [https://en.wikipedia.org/wiki/Amyloid_beta Amyloid Beta] (Aβ) peptides which are products of the cleavage of [https://en.wikipedia.org/wiki/Amyloid-beta_precursor_protein#:~:text=Amyloid%2Dbeta%20precursor%20protein%20(APP,antimicrobial%20activity%2C%20and%20iron%20export. Human Amyloid-Beta Precursor Protein] (hAPP) <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. Within the cells, there is an accumulation of hyperphosphorylated [https://en.wikipedia.org/wiki/Tau_protein Tau] protein. Research has shown that the concentration of cholesterol within cells can affect the production of Aβ <ref name "Chang">doi:10.1002/iub.305</ref>. As the concentration of cholesterol in the endoplasmic reticulum of neurons increases, hAPP is downregulated <ref name "Chang">doi:10.1002/iub.305</ref>. Inhibition of ACAT1 would lead to higher concentrations of cholesterol in the cells, signaling downregulation of hAPP. Less hAPP available decreases the amount of Aβ peptides being produced which then reduces the available Aβ peptides that form the extracellular plaques associated with Alzheimer's Disease <ref name "Chang">doi:10.1002/iub.305</ref> <ref name="Shibuya"> PMID:26669800</ref>. |
| - | ===Other Diseases=== | ||
| - | ACAT is also involved in diseases such as [https://www.mayoclinic.org/diseases-conditions/parkinsons-disease/symptoms-causes/syc-20376055 Parkinson’s Disease] and other neurodegenerative diseases due to the accumulation of Aβ plaques in the brain. After research on [https://www.mayoclinic.org/diseases-conditions/glioma/symptoms-causes/syc-20350251 glioma], [https://www.mayoclinic.org/diseases-conditions/prostate-cancer/symptoms-causes/syc-20353087 prostate cancer], [https://www.mayoclinic.org/diseases-conditions/pancreatic-cancer/symptoms-causes/syc-20355421 pancreatic cancer], [https://www.mayoclinic.org/diseases-conditions/leukemia/symptoms-causes/syc-20374373 leukemia], and [https://www.mayoclinic.org/diseases-conditions/breast-cancer/symptoms-causes/syc-20352470 breast cancer], it has been noted that ACAT plays a role in the progression of cancer over time. Recently, Ayyagari et al. found that there was a significant increase in ACAT-1 expression in ovarian cancer cell lines <ref name="Ayyagari"> doi:10.1371/journal.pone.0228024</ref>. ACAT-2 is believed to be upregulated in Nephrotic Syndrome (NS) which can lead to cardiovascular disease and renal diseases <ref name="Vaziri"> doi:10.1161/01.CIR.0000136023.70841.0F</ref>. Because of ACAT's activity in tissues such as the aorta, intestine, and liver, it plays a role in Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Studies have shown that the inhibition of ACAT-2 can slow the progression of Atherosclerosis <ref name="Willner"> doi:10.1073/pnas.0336398100</ref>. Guan discussed a previous study which found that CI-976 decreased the size of atherosclerosis plaques and the overall concentration of cholesterol in the blood plasma of animals that had been fed a high cholesterol diet <ref name="Guan"> doi:10.1038/s41467-020-16288-4</ref> | ||
| + | </StructureSection> | ||
| - | </StructureSection> | ||
== References == | == References == | ||
| - | ACAT article <ref name=”Qian”>PMID:32433614</ref> | ||
| - | SOAT Article <ref name=”Shengcheng”>PMID:32424158</ref> | ||
<references/> | <references/> | ||
==Student Contributors== | ==Student Contributors== | ||
| - | *Haylie Moehlenkamp | + | * Haylie Moehlenkamp, Megan Fleshman, Tori Templin |
Current revision
Acyl-Coenzyme A: Cholesterol Acetyltransferase 1 (ACAT1)
| |||||||||||
References
- ↑ Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, Du X, Yang H, Wong CCL, Yan N. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020 May;581(7808):333-338. doi: 10.1038/s41586-020-2290-0. Epub 2020 May, 13. PMID:32433614 doi:http://dx.doi.org/10.1038/s41586-020-2290-0
- ↑ Farese RV Jr. The nine lives of ACAT inhibitors. Arterioscler Thromb Vasc Biol. 2006 Aug;26(8):1684-6. doi:, 10.1161/01.ATV.0000227511.35456.90. PMID:16857957 doi:http://dx.doi.org/10.1161/01.ATV.0000227511.35456.90
- ↑ 3.0 3.1 3.2 3.3 3.4 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ 4.0 4.1 4.2 Guan C, Niu Y, Chen SC, Kang Y, Wu JX, Nishi K, Chang CCY, Chang TY, Luo T, Chen L. Structural insights into the inhibition mechanism of human sterol O-acyltransferase 1 by a competitive inhibitor. Nat Commun. 2020 May 18;11(1):2478. doi: 10.1038/s41467-020-16288-4. PMID:32424158 doi:http://dx.doi.org/10.1038/s41467-020-16288-4
- ↑ Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-An in vitro study. PLoS One. 2020 Jan 24;15(1):e0228024. doi: 10.1371/journal.pone.0228024., eCollection 2020. PMID:31978092 doi:http://dx.doi.org/10.1371/journal.pone.0228024
- ↑ Vaziri ND, Liang KH. Acyl-coenzyme A:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004 Jul 27;110(4):419-25. doi: 10.1161/01.CIR.0000136023.70841.0F. , Epub 2004 Jul 19. PMID:15262831 doi:http://dx.doi.org/10.1161/01.CIR.0000136023.70841.0F
- ↑ Willner EL, Tow B, Buhman KK, Wilson M, Sanan DA, Rudel LL, Farese RV Jr. Deficiency of acyl CoA:cholesterol acyltransferase 2 prevents atherosclerosis in apolipoprotein E-deficient mice. Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):1262-7. doi: 10.1073/pnas.0336398100., Epub 2003 Jan 21. PMID:12538880 doi:http://dx.doi.org/10.1073/pnas.0336398100
- ↑ 8.0 8.1 Shibuya Y, Chang CC, Chang TY. ACAT1/SOAT1 as a therapeutic target for Alzheimer's disease. Future Med Chem. 2015;7(18):2451-67. doi: 10.4155/fmc.15.161. Epub 2015 Dec 15. PMID:26669800 doi:http://dx.doi.org/10.4155/fmc.15.161
Student Contributors
- Haylie Moehlenkamp, Megan Fleshman, Tori Templin