Hexokinase
From Proteopedia
| (6 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
__TOC__ | __TOC__ | ||
== Function == | == Function == | ||
| - | '''Hexokinase''' is an enzyme that phosphorylates a six-carbon sugar, a hexose, to a hexose phosphate. In most tissues and organisms, glucose is the most important substrate of hexokinases, and glucose 6-phosphate the most important product. Hexokinases have been found in every organism checked, ranging from bacteria, yeast, and plants, to humans and other vertebrates. They are categorized as actin fold proteins, sharing a common ATP binding site core surrounded by more variable sequences that determine substrate affinities and other properties. Several hexokinase isoforms or isozymes providing different functions can occur in a single species. See [[Glycolysis Enzymes]]. | + | '''Hexokinase''' is an enzyme that phosphorylates a six-carbon sugar, a hexose, to a hexose phosphate. In most tissues and organisms, glucose is the most important substrate of hexokinases, and glucose 6-phosphate the most important product. Hexokinases have been found in every organism checked, ranging from bacteria, yeast, and plants, to humans and other vertebrates. They are categorized as actin fold proteins, sharing a common ATP binding site core surrounded by more variable sequences that determine substrate affinities and other properties. Several hexokinase isoforms or isozymes providing different functions can occur in a single species. See [[Glycolysis Enzymes]], [[Glycogenesis]]. |
* '''Hexokinase I/A''' is found in all mammalian tissues, and is considered a "housekeeping enzyme," unaffected by most physiological, hormonal, and metabolic changes. More details in [[Hexokinase Type 1]]. | * '''Hexokinase I/A''' is found in all mammalian tissues, and is considered a "housekeeping enzyme," unaffected by most physiological, hormonal, and metabolic changes. More details in [[Hexokinase Type 1]]. | ||
| Line 12: | Line 12: | ||
* '''Hexokinase IV/D''' is also known as '''glucokinase'''. | * '''Hexokinase IV/D''' is also known as '''glucokinase'''. | ||
| - | Additional details in [[The Structure and Mechanism of Hexokinase]] and [[Conformational changes in proteins]] (in Spanish). | + | Additional details in [[The Structure and Mechanism of Hexokinase]], [[Glucokinase and Phosphorylase. Conformational changes (Spanish)]] and [[Conformational changes in proteins]] (in Spanish). |
== Structure of Hexokinase == | == Structure of Hexokinase == | ||
| Line 21: | Line 21: | ||
== Conformational change associated with substrate binding == | == Conformational change associated with substrate binding == | ||
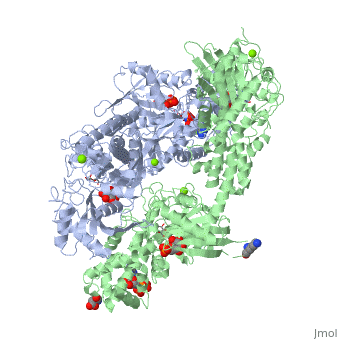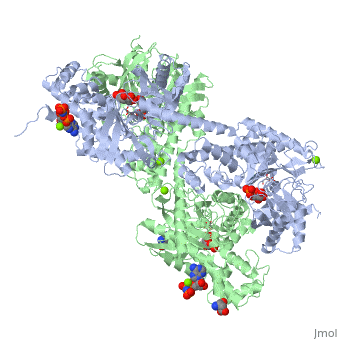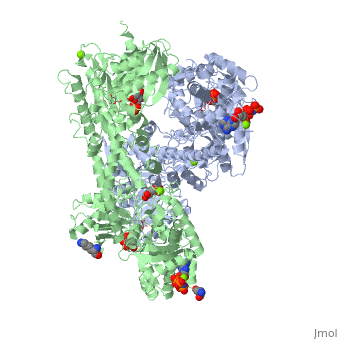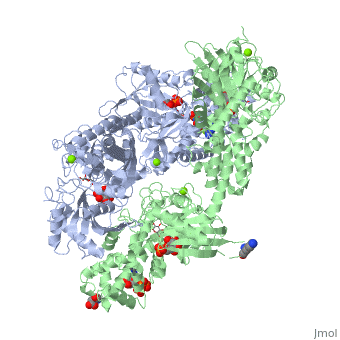
| - | When hexokinase binds to glucose (one of its two substrates), it exhibits induced fit. This means that in the open form of the enzyme, the binding site is not fully formed. Upon binding glucose, hexokinase switches into a closed form, excluding aqueous solvent from the substrate. This is illustrated here <scene name='45/452482/Induced_fit/ | + | When hexokinase binds to glucose (one of its two substrates), it exhibits induced fit. This means that in the open form of the enzyme, the binding site is not fully formed. Upon binding glucose, hexokinase switches into a closed form, excluding aqueous solvent from the substrate. This is illustrated here <scene name='45/452482/Induced_fit/2'>comparing the structures of free and glucose-bound hexokinase</scene>. |
<jmol> | <jmol> | ||
| Line 28: | Line 28: | ||
<script>anim off; delay 0.5; model 1</script> | <script>anim off; delay 0.5; model 1</script> | ||
<text>Open</text> | <text>Open</text> | ||
| + | <checked>true</checked> | ||
</item> | </item> | ||
<item> | <item> | ||
<script>anim off; delay 0.5; model 2</script> | <script>anim off; delay 0.5; model 2</script> | ||
<text>Close</text> | <text>Close</text> | ||
| + | <checked>false</checked> | ||
</item> | </item> | ||
<item> | <item> | ||
<script>anim mode palindrome; anim on</script> | <script>anim mode palindrome; anim on</script> | ||
<text>Animate</text> | <text>Animate</text> | ||
| - | <checked> | + | <checked>false</checked> |
</item> | </item> | ||
</jmolRadioGroup> | </jmolRadioGroup> | ||
| Line 43: | Line 45: | ||
It is a bit easier to see the extent and nature of the changes in this <jmol> | It is a bit easier to see the extent and nature of the changes in this <jmol> | ||
<jmolLink> | <jmolLink> | ||
| - | <script> script "/wiki/images/a/a2/Storymorph.spt"; | + | <script> script "/wiki/images/a/a2/Storymorph.spt"; model 2; color background black; |
structures = [{1.1},{1.2}]; | structures = [{1.1},{1.2}]; | ||
domain2 = {82-210}; | domain2 = {82-210}; | ||
| Line 52: | Line 54: | ||
<text>morph</text> | <text>morph</text> | ||
</jmolLink> | </jmolLink> | ||
| - | </jmol>. | + | </jmol> <ref>The [[Jmol/Storymorph|Storymorph Jmol scripts]] creates the interpolated coordinates of the morph on the fly.</ref>. |
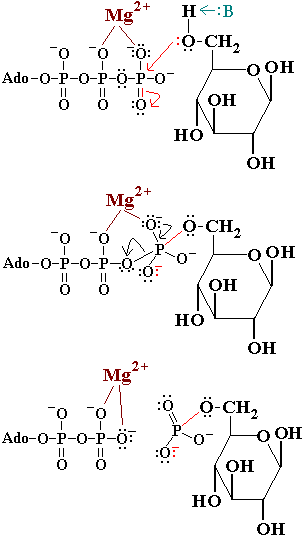
== Mechanism of Hexokinase == | == Mechanism of Hexokinase == | ||
| Line 88: | Line 90: | ||
8.↑ Aleshin A, Malfois M, Liu X, Kim C, Fromm H, Honzatko R, Koch M, Svergun D. Nonaggregating Mutant of Recombinant Human Hexokinase I Exhibits Wild-Type Kinetics and Rod-like Conformations in Solution. Biochem. 1999 Apr 29;38:8359-8366. | 8.↑ Aleshin A, Malfois M, Liu X, Kim C, Fromm H, Honzatko R, Koch M, Svergun D. Nonaggregating Mutant of Recombinant Human Hexokinase I Exhibits Wild-Type Kinetics and Rod-like Conformations in Solution. Biochem. 1999 Apr 29;38:8359-8366. | ||
| - | + | <references/> | |
Seth Bawel and Kyle_Schroering created this page in Che 361 at Wabash College. | Seth Bawel and Kyle_Schroering created this page in Che 361 at Wabash College. | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Additional Resources
For additional information, see: Carbohydrate Metabolism
References
1.↑ Pollard-Knight D, Cornish-Bowden A. Mechanism of liver glucokinase. Mol Cell Biochem. 1982 Apr 30;44(2):71-80. PMID:7048063
2.↑ 2.0 2.1 Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structural basis for allosteric regulation of the monomeric allosteric enzyme human glucokinase. Structure. 2004 Mar;12(3):429-38. PMID:15016359 doi:10.1016/j.str.2004.02.005
3.↑ Postic C, Shiota M, Magnuson MA. Cell-specific roles of glucokinase in glucose homeostasis. Recent Prog Horm Res. 2001;56:195-217. PMID:11237213
4.↑ Zeng C, Aleshin A, Hardie J, Harrison R, Fromm H. ATP-Binding site of Human Brain Hexokinase as Studied by Molecular Modeling and Site-Directed Mutagenesis. Biochem. 1996 Aug 6;35:13157-13164.
5.↑ hammes G, and Kochavi D. Studies of the Enzyme Hexokinase: Kinetic Inhibition by Products. Massachusetts Institute of Technology. 1961 Oct 5.
6.↑ Ralph E, Thomson J, Almaden J, Sun S. Glucose Modulation fo Glucokinase Activation by Small Molecules. Biochem. 2008 Feb 15;47:5028-5036.
7.↑ Pal P, and Miller B. Activating Mutations in the Human Glucokinase Gene Revealed by Genetic Selection. Biochem. 2008 Dec 3;48:814-816.
8.↑ Aleshin A, Malfois M, Liu X, Kim C, Fromm H, Honzatko R, Koch M, Svergun D. Nonaggregating Mutant of Recombinant Human Hexokinase I Exhibits Wild-Type Kinetics and Rod-like Conformations in Solution. Biochem. 1999 Apr 29;38:8359-8366.
- ↑ The Storymorph Jmol scripts creates the interpolated coordinates of the morph on the fly.
Seth Bawel and Kyle_Schroering created this page in Che 361 at Wabash College.
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Karsten Theis, Ann Taylor, Alexander Berchansky