Histone acetyltransferase 1-2 Complex (HAT1/2)
From Proteopedia
(Difference between revisions)
(greek letter in green link breaks code to be sent to JSmol) |
|||
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | {{BAMBED | ||
| + | |DATE=June 14, 2021 | ||
| + | |OLDID=3412172 | ||
| + | |BAMBEDDOI=10.1002/bmb.21759 | ||
| + | }} | ||
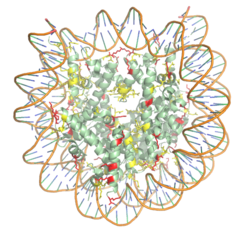
=The Yeast HAT1-HAT2 Histone Acetyltransferase Complex Bound to the Histone H4 substrate= | =The Yeast HAT1-HAT2 Histone Acetyltransferase Complex Bound to the Histone H4 substrate= | ||
<StructureSection load='4PSW' size='350' frame='true' side='right' caption='HAT1-HAT2 Complex pdb: [[4psw]]' scene='83/834210/Overview/2'> | <StructureSection load='4PSW' size='350' frame='true' side='right' caption='HAT1-HAT2 Complex pdb: [[4psw]]' scene='83/834210/Overview/2'> | ||
| Line 21: | Line 26: | ||
===Acetyl-CoA Binding Site=== | ===Acetyl-CoA Binding Site=== | ||
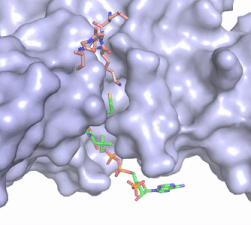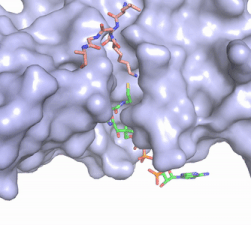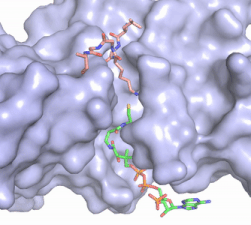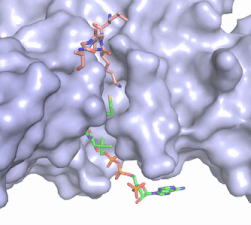
| - | [[Image:Hat1.gif|400 px|left|thumb|Figure 2. Coenzyme A (CoA, green sticks) and the histone H4 peptide substrate (pink sticks) are shown in the binding groove of HAT1. The movie was made in PyMol using 4psw.pdb, then converted to gif format using EZgif.]] The CoA molecule is buried in a deep channel in the HAT1 subunit, where the free rotation of several bonds in the [https://en.wikipedia.org/wiki/Pantetheine pantetheine] group give the molecule a bent conformation (Figure 2). The conserved Arg/Gln-X-X-Gly-X-Gly/Ala <scene name='83/834210/Newcoa/11'>signature sequence</scene> (residues 227-232) of CoA containing proteins wraps around the 5'-diphosphate moiety of the CoA. This motif also positions the negatively charged β-phosphate group of CoA at the N-terminal dipole of the helix spanning residues 230-245, further stabilizing the interaction. The <scene name='83/834210/Hydrophobic_pocket/ | + | [[Image:Hat1.gif|400 px|left|thumb|Figure 2. Coenzyme A (CoA, green sticks) and the histone H4 peptide substrate (pink sticks) are shown in the binding groove of HAT1. The movie was made in PyMol using 4psw.pdb, then converted to gif format using EZgif.]] The CoA molecule is buried in a deep channel in the HAT1 subunit, where the free rotation of several bonds in the [https://en.wikipedia.org/wiki/Pantetheine pantetheine] group give the molecule a bent conformation (Figure 2). The conserved Arg/Gln-X-X-Gly-X-Gly/Ala <scene name='83/834210/Newcoa/11'>signature sequence</scene> (residues 227-232) of CoA containing proteins wraps around the 5'-diphosphate moiety of the CoA. This motif also positions the negatively charged β-phosphate group of CoA at the N-terminal dipole of the helix spanning residues 230-245, further stabilizing the interaction. The <scene name='83/834210/Hydrophobic_pocket/3'>beta-mercaptoethylamine group</scene> of CoA rests in the hydrophobic pocket formed by the side chains of residues Ile-217,Pro-257 and Phe-261. In the HAT1-Acetyl coenzyme A structure determined without HAT2 and the H4 substrate (PDB: 1bob) the main chain atoms of <scene name='83/834210/Acetylcoa_structure/3'>Phe220</scene> were revealed to play a critical role in binding to acetyl-CoA. The carbonyl oxygen of Phe220 hydrogen bonds to the amine of the β-mercaptoethylamine group of the co-factor, while the Phe220 amide nitrogen hydrogen bonds to the acetyl group of acetyl-CoA.<ref name=”Dutnall”>PMID:10384314</ref> The latter interaction has important implications for the mechanism as described below. |
| + | |||
= Mechanism = | = Mechanism = | ||
| Line 27: | Line 33: | ||
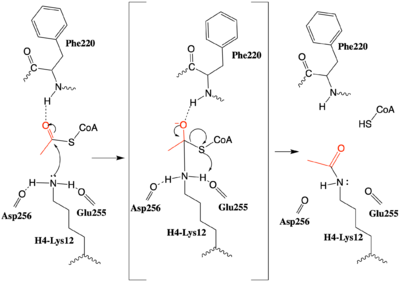
After many structural studies, the complete catalytic mechanism for HAT1 remains unclear. In particular, the identity of the general base needed to deprotonate the substrate lysine is uncertain. In a previous study a structural overlay of HAT1 and Gcn5, a better-understood HAT enzyme, found a conserved glutamate residue in the active site of both enzymes. Mutation of this glutamate (equivalent to Glu255 in 2psw.pdb) was shown to decrease the catalytic ability of HAT1, identifying it to be important for catalysis. <ref name="Yang"/> Given the proximity of <scene name='83/834210/Mechanism_glu_lys_coa/4'>Glu255 and Asp256</scene> this mechanism could be supported by structure of the HAT1-HAT2 complex with histone H4, however it would require a 180° shift in the direction of the side chains to act as a general base that deprotonates H4-Lys12, enhancing its nucleophilic character. | After many structural studies, the complete catalytic mechanism for HAT1 remains unclear. In particular, the identity of the general base needed to deprotonate the substrate lysine is uncertain. In a previous study a structural overlay of HAT1 and Gcn5, a better-understood HAT enzyme, found a conserved glutamate residue in the active site of both enzymes. Mutation of this glutamate (equivalent to Glu255 in 2psw.pdb) was shown to decrease the catalytic ability of HAT1, identifying it to be important for catalysis. <ref name="Yang"/> Given the proximity of <scene name='83/834210/Mechanism_glu_lys_coa/4'>Glu255 and Asp256</scene> this mechanism could be supported by structure of the HAT1-HAT2 complex with histone H4, however it would require a 180° shift in the direction of the side chains to act as a general base that deprotonates H4-Lys12, enhancing its nucleophilic character. | ||
| - | An alternative mechanism (Figure 3) that is better supported by the HAT1-HAT2-histone H4 structure, proposes that the attacking lysine would be deprotonated upon entry into active site due the proximity of the acidic side chains of Glu255 and Asp256. Additionally, there are <scene name='83/834210/Mechanism_lys_carbonyls/3'>three carbonyl oxygens</scene> in the main chain of Ser218, Glu255, and Asp256 which are in hydrogen bonding distance with the ε-amine of the attacking lysine. They will act to withdraw positive charge from the attacking nitrogen improving its nucleophilic nature and perhaps better orient the lone pair electrons for nucleophillic attack. Additionally, the interaction of the Phe220 amide with the carbonyl oxygen of the acetyl group enhances the electrophilic nature the carbonyl carbon being attacked. In the second step of the reaction, the lone pair on the lysine attacks the carbonyl carbon of acetyl-CoA, forming an oxyanion containing tetrahedral transition state. The structure does not definitively reveal residues in an oxyanion hole that stabilize the transition state | + | [[Image:HAT1_mechanism.png|400px|right|thumb|Figure 3: The proposed HAT1 Mechanism with the transferred acetyl group in red. (The carbonyl of Ser218 described in the text and <scene name='83/834210/Mechanism_lys_carbonyls/1'>green link</scene> is not shown activating the Lys12 nucleophile)]]An alternative mechanism (Figure 3) that is better supported by the HAT1-HAT2-histone H4 structure, proposes that the attacking lysine would be deprotonated upon entry into active site due the proximity of the acidic side chains of Glu255 and Asp256. Additionally, there are <scene name='83/834210/Mechanism_lys_carbonyls/3'>three carbonyl oxygens</scene> in the main chain of Ser218, Glu255, and Asp256 which are in hydrogen bonding distance with the ε-amine of the attacking lysine. They will act to withdraw positive charge from the attacking nitrogen improving its nucleophilic nature and perhaps better orient the lone pair electrons for nucleophillic attack. Additionally, the interaction of the Phe220 amide with the carbonyl oxygen of the acetyl group enhances the electrophilic nature the carbonyl carbon being attacked. In the second step of the reaction, the lone pair on the lysine attacks the carbonyl carbon of acetyl-CoA, forming an oxyanion containing tetrahedral transition state. The structure does not definitively reveal residues in an oxyanion hole that stabilize the transition state but superposition of the HAT1-acetyl coenzyme A structure with the HAT1-HAT2-H4 substrate structure suggests <scene name='83/834210/Mechanism_final/5'>the main chain amide of Phe220</scene>, which binds the carbonyl oxygen of the acetyl group before attack, is a likely candidate. Finally, upon electron reorganization, the C-S scissile bond breaks leaving the H4Lys12 acetylated and Coenzyme A as products. |
= Inhibition = | = Inhibition = | ||
| Line 39: | Line 45: | ||
= Student Contributors = | = Student Contributors = | ||
Morgan Buckley, Jordan Finch, Caitlin Gaich, Kiran Kaur, Emily Leiderman, Ben Nick | Morgan Buckley, Jordan Finch, Caitlin Gaich, Kiran Kaur, Emily Leiderman, Ben Nick | ||
| + | |||
| + | [[Category:Featured in BAMBED]] | ||
Current revision
This page, as it appeared on June 14, 2021, was featured in this article in the journal Biochemistry and Molecular Biology Education.
The Yeast HAT1-HAT2 Histone Acetyltransferase Complex Bound to the Histone H4 substrate
| |||||||||||
Student Contributors
Morgan Buckley, Jordan Finch, Caitlin Gaich, Kiran Kaur, Emily Leiderman, Ben Nick
Proteopedia Page Contributors and Editors (what is this?)
Mark Macbeth, Angel Herraez, Michal Harel, Valentine J Klimkowski