Esterification
From Proteopedia
(Difference between revisions)
(New page: ==Your Heading Here (maybe something like 'Structure')== <StructureSection load='1stp' size='340' side='right' caption='Caption for this structure' scene=''> This is a default text for you...) |
|||
| (49 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='350' side='right' caption='Esterification of fatty acid with ethanol' scene='88/887592/Esterification_with_fifth_step/1'> | |
| - | <StructureSection load=' | + | Esterification is a chemical reaction of an [http://en.wikipedia.org/wiki/Acid acid] with an [http://en.wikipedia.org/wiki/Alcohol alcohol] (R'OH) to form an ester (RCOOR'). |
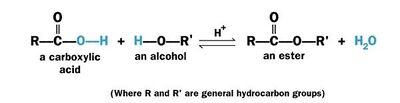
| - | + | Usually esterification refers to reaction between an organic (carboxylic) acid (RCOOH) with an alcohol (R'OH) to form an ester (RCOOR') and water and called '''Fischer esterification'''. | |
| - | + | The chemical reaction for Fischer esterification is given below: | |
| + | [[Image:Esterformation2.jpg|400px]] | ||
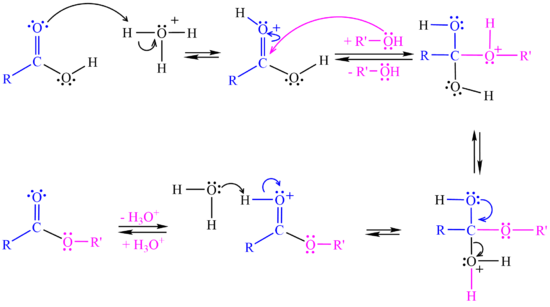
| - | + | The <jmol><jmolLink><script>anim mode once; frame range 1 10; delay 0.5; frame play</script><text>reaction starts</text></jmolLink></jmol> when the carboxylic acid accepts a proton from the strong acid catalyst. | |
| + | In the <jmol><jmolLink><script>anim mode once; frame range 11 19; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol> the alcohol attacks the protonated carbonyl group to create a tetrahedral intermediate structure. | ||
| + | In the <jmol><jmolLink><script>anim mode once; frame range 20 41; delay 0.5; frame play</script><text>third step</text></jmolLink></jmol> a proton is lost at one oxygen atom and bonds to another oxygen atom. | ||
| + | In the <jmol><jmolLink><script>anim mode once; frame range 41 50; delay 0.5; frame play</script><text>fourth step</text></jmolLink></jmol> a water molecule leaves the structure. | ||
| + | In the <jmol><jmolLink><script>anim mode once; frame range 51 59; delay 0.5; frame play</script><text>fifth step</text></jmolLink></jmol> a proton (H+) leaves the carbonyl group, transfers to a base and '''ester''' is formed. | ||
| - | + | [[Image:Esterification Mechanism.png|550px]] <br> | |
| + | An animated example of this reaction is shown. Please click on the buttons below to '''animate''' the reaction with different representations. Use the '''popup''' button to enlarge the view and the '''quality''' button to turn on anti-aliasing. | ||
| - | + | <jmol> | |
| + | |||
| + | <jmolButton><script>frame 1</script><text>First</text></jmolButton> | ||
| + | <jmolButton><script>frame prev</script><text>Previous</text></jmolButton> | ||
| + | <jmolButton><script>anim mode once; frame 1; delay 0.5; anim on</script><text>Play</text></jmolButton> | ||
| + | <jmolButton><script>frame next</script><text>Next</text></jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | <jmol> | ||
| + | <jmolButton><script>frame all</script><text>All frames</text></jmolButton> | ||
| + | <jmolButton><script>animation mode palindrome 0.5 0.2; anim on</script><text>Loop backwards and forward</text></jmolButton> | ||
| + | <jmolButton><script>if(_animating);anim off;else;frame play;endif</script><text>Toggle animation</text></jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | <jmol> | ||
| + | <jmolButton><script>spacefill 90; dots on; wireframe 30;</script><text>translucent atoms</text></jmolButton> | ||
| + | <jmolButton><script>spacefill on; dots off;</script><text>spacefilling atoms</text></jmolButton> | ||
| + | <jmolButton><script>spacefill 90; dots off; wireframe 30;</script><text>backbone</text></jmolButton> | ||
| + | </jmol> | ||
| + | |||
| + | The animation was originally done by [[User:Verena Pietzner | Prof. Dr. Verena Pietzner]]; for details, see her web site ChiLe<ref>[http://www.chemieunterricht-interaktiv.de/en/index.html ChiLe Web Site]</ref>. The implementation into Proteopedia was done by [[User:Jaime Prilusky | Prof. Jaime Prilusky]], [[User:Joel L. Sussman | Prof. Joel L. Sussman ]] | ||
| + | and [[User:Veronika Pelekhov | Veronika Pelekhov]]. | ||
| - | == Structural highlights == | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
| + | ===See also=== | ||
| + | [[SN1_reaction|S<sub>N</sub>1 reaction: Substitution of Cl<sup>−</sup> and ''tert''-Butanol ]]<br> | ||
| + | [[SN2_reaction|S<sub>N</sub>2 reaction: substitution of Cl<sup>−</sup> and methanol ]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
SN2 reaction: substitution of Cl− and methanol