This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
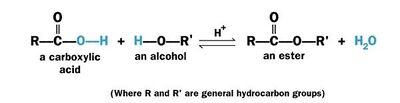
Esterification
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 8: | Line 8: | ||
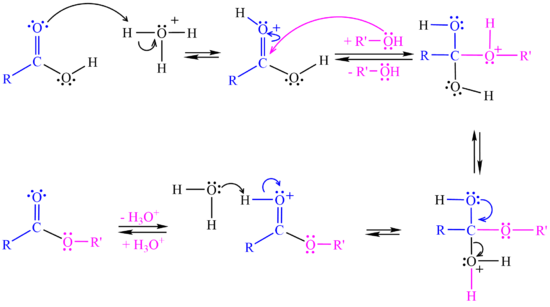
In the <jmol><jmolLink><script>anim mode once; frame range 11 19; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol> the alcohol attacks the protonated carbonyl group to create a tetrahedral intermediate structure. | In the <jmol><jmolLink><script>anim mode once; frame range 11 19; delay 0.5; frame play</script><text>second step</text></jmolLink></jmol> the alcohol attacks the protonated carbonyl group to create a tetrahedral intermediate structure. | ||
In the <jmol><jmolLink><script>anim mode once; frame range 20 41; delay 0.5; frame play</script><text>third step</text></jmolLink></jmol> a proton is lost at one oxygen atom and bonds to another oxygen atom. | In the <jmol><jmolLink><script>anim mode once; frame range 20 41; delay 0.5; frame play</script><text>third step</text></jmolLink></jmol> a proton is lost at one oxygen atom and bonds to another oxygen atom. | ||
| - | In the <jmol><jmolLink><script>anim mode once; frame range 41 50; delay 0.5; frame play</script><text>fourth step</text></jmolLink></jmol> water molecule leaves the structure. | + | In the <jmol><jmolLink><script>anim mode once; frame range 41 50; delay 0.5; frame play</script><text>fourth step</text></jmolLink></jmol> a water molecule leaves the structure. |
| - | In the <jmol><jmolLink><script>anim mode once; frame range 51 59; delay 0.5; frame play</script><text>fifth step</text></jmolLink></jmol> . | + | In the <jmol><jmolLink><script>anim mode once; frame range 51 59; delay 0.5; frame play</script><text>fifth step</text></jmolLink></jmol> a proton (H+) leaves the carbonyl group, transfers to a base and '''ester''' is formed. |
[[Image:Esterification Mechanism.png|550px]] <br> | [[Image:Esterification Mechanism.png|550px]] <br> | ||
Current revision
| |||||||||||
See also
SN1 reaction: Substitution of Cl− and tert-Butanol
SN2 reaction: substitution of Cl− and methanol