We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 1697
From Proteopedia
(Difference between revisions)
| (39 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | {{Sandbox_Reserved_BHall_F21}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | + | <scene name='89/892740/7kir_catalytic_amino_acids/1'>Text To Be Displayed</scene><scene name='89/892740/Protein_view_2/6'>Text To Be Displayed</scene>{{Sandbox_Reserved_BHall_F21}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> |
| - | == | + | ==7KIR== |
| - | <StructureSection load=' | + | <StructureSection load='7KIR' size='340' side='right' caption='Inositol plyphosphate 1-phosphatase' scene=''> |
This is a default text for your page '''Sandbox 1677'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page '''Sandbox 1677'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref>PMID:21638687</ref> to the rescue. | ||
== Function of your protein == | == Function of your protein == | ||
| - | The | + | The protein 7KIR is found in the organism Bos taurus, is a a protype member of proteins dealing with inositol signaling. The function of <scene name='89/892740/Protein_view_2/9'>7KIR</scene> is dephosphorylating inositol, used in inositol signaling, the substrate of the enzyme is IP3 and the product is IP2. The amino acids of the protein hold onto the magnesium metal ions that then hold onto the water molecule, this allows for it to take off a phosphate. |
== Biological relevance and broader implications == | == Biological relevance and broader implications == | ||
| + | This protein is involved in dephosphorylation of inositol which inhibits inositol signaling in cattle. The metal ions assist in holding the water so that it can attack the phosphate to take it off. The two metal ions, Magnesium and Lithium, are particularly important in activation and inhibition of inositol signaling. Since these enzymes are seen in many different organisms, with slight mutations in the motif of the enzyme without change in function, understanding the mechanics of enzyme inhibition in this metabolic pathway would allow a better understanding of metabolic pathways. | ||
== Important amino acids== | == Important amino acids== | ||
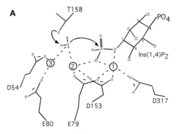
| + | In the protein the substrate is <scene name='89/892740/Ligand_view/1'>D-MYO-INOSITOL-1,4-BISPHOSPHATE</scene>, will be dephosphorylated by the water brought in by the magnesium ions.In this protein the amino acids do not interact with the substrate itself, instead they interact with the metal ions. When magnesium is present, and the protein therefore activated, the amino acids will hold onto the magnesium ions. These magnesium ions that interact with the inositol and water so that it can dephosphorylate the substrate. The motif of enzymes in this family is DPIDXT. In this particular protein the motif is <scene name='89/892740/Motif_view/1'>D54, E80, E79, D153, and D317</scene>. There are some mutations in the enzyme but the overall function of the protein is still the same. | ||
== Structural highlights == | == Structural highlights == | ||
| + | The protein has thirteen structural elements. The amino acids in the bind to the substrate all participate in hydrogen bonding to the substrate. The structure is <scene name='89/892740/Secondary_structures/1'>77% helix and 23% beta sheet</scene>, the betta sheets allow for twisting of the molecule so that the substrate better fits within the enzyme. The helices allow for hydrogen bonding throughout to stabilize the structure with assistance from metal ions; specifically magnesium ions. <ref> PMID 33172890 </ref> | ||
| + | There is no quaternary structures in the protein but several <scene name='89/892740/7kir_quaternary_structures/1'>tertiary structures</scene> that play an important role in the structure of the protein and therefore its function. There are many covalent bonds due to hydrophobic and nonpolar amino acids that form the structure. The amino acids involved with the <scene name='89/892740/Quat_structure_catalytic_amino/1'>catalytic amino acids are mostly polar</scene>, the magnesium ions assist in pulling the enzyme into shape that allow for it to be in the <scene name='89/892740/Spacefill_7kir/1'>proper shape</scene> so that it can dephosphorylate the inositol. It wraps around the magnesium ions so that it can better attach to the water and then attack the phosphate on inositol. When the lithium ions interact with the enzyme a shape change occurs that will not allow for the magnesium ions to attach to the enzyme, and therefore the water molecules. | ||
| + | The rest of the enzyme is circled around the substrate which allows for a better ability to bind to the substrate, it is shown here how the <scene name='89/892740/Hydrophobic_effect/1'>hydrophobic effect</scene> how the nonpolar and polar amino acids allow for it to have this shape. | ||
== Other important features == | == Other important features == | ||
| + | The protein 7KIR is a protype member of proteins that work in inositol signaling, specifically dephosphorylating inositol to stop inositol signaling. The protein that the paper primarily mentions is <scene name='89/892740/1inp/1'>1INP</scene>, all of the proteins in this family share a motif, DPID X T. The X in the motif means that there can be any amino acid in there, in 1INP it is alanine while in <scene name='89/892740/7kir_catalytic_amino_acids/1'>7KIR</scene> is aspartic acid. The mutation in these proteins have no functional impact on the protein's ability to dephosphorylate inositol. | ||
| + | One of the unique things of this enzyme is its use of metal ions, the <scene name='89/892740/Interact_with_metal_ions/1'>amino acids bond with the metal ions</scene> that then bind with the water. The water attacks the phosphate which causes the inositol to dephosphorylate the substrate as seen in the image to the right. The catalytic amino acids in enzyme are mainly polar which causes the magnesium ions to better bond with them. | ||
| + | [[Image:Pic.png | thumb]] | ||
This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
Current revision
| This Sandbox is Reserved from 10/01/2021 through 01/01//2022 for use in Biochemistry taught by Bonnie Hall at Grand View University, Des Moines, USA. This reservation includes Sandbox Reserved 1690 through Sandbox Reserved 1699. |
To get started:
More help: Help:Editing |
7KIR
| |||||||||||
References
- ↑ Hanson, R. M., Prilusky, J., Renjian, Z., Nakane, T. and Sussman, J. L. (2013), JSmol and the Next-Generation Web-Based Representation of 3D Molecular Structure as Applied to Proteopedia. Isr. J. Chem., 53:207-216. doi:http://dx.doi.org/10.1002/ijch.201300024
- ↑ Herraez A. Biomolecules in the computer: Jmol to the rescue. Biochem Mol Biol Educ. 2006 Jul;34(4):255-61. doi: 10.1002/bmb.2006.494034042644. PMID:21638687 doi:10.1002/bmb.2006.494034042644
- ↑ Dollins DE, Xiong JP, Endo-Streeter S, Anderson DE, Bansal VS, Ponder JW, Ren Y, York JD. A Structural Basis for Lithium and Substrate Binding of an Inositide Phosphatase. J Biol Chem. 2020 Nov 10. pii: RA120.014057. doi: 10.1074/jbc.RA120.014057. PMID:33172890 doi:http://dx.doi.org/10.1074/jbc.RA120.014057